Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2020, vol. 19(1–2), pp. 55–61 DOI: https://doi.org/10.15388/LietChirur.2020.19.26
Primary Myeloid Sarcoma of the Ileum and Mesentery Causing Small Bowel Obstruction: Case Report and Literature Review
Andrej Nikolovski
University Surgical Clinic “St. Naum Ohridski”, Skopje, North Macedonia
E-mail: andrejnikolovski@ymail.com
Dragoslav Mladenovikj
University Surgical Clinic “St. Naum Ohridski”, Skopje, North Macedonia
E-mail: dmladenovik@hotmail.com
Aleksandra Veljanovska
University Clinic for Hematology, Skopje, North Macedonia
E-mail: aleksandrapivkova@yahoo.com
Gordana Petrusevka
Institute for Pathology, Medical Faculty, Skopje, North Macedonia
E-mail: gordanap61@yahoo.com
Abstract. Myeloid sarcoma (extramedullary myeloblastoma, granulocytic sarcoma, chloroma) is an extramedullary isolated malignant tumor of myeloblasts and immature myelocytes. It can occur anywhere in the body as a solitary tumor or can be accompanied with acute myeloid leukemia. We are presenting a case of a young male patient that presented with sings of a small bowel obstruction and a palpable tumor mass in the abdomen. After uneventful postoperative period, the immunohistochemistry analysis reported an extramedullary myeloid sarcoma since a normal bone marrow biopsy was revealed.
Key words: primary myeloid sarcoma, small bowel obstruction.
Received: 2020/03/02. Accepted: 2020/04/14.
Copyright © 2020 Andrej Nikolovski, Dragoslav Mladenovikj, Aleksandra Veljanovska, Gordana Petrusevka. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Myeloid sarcoma (extramedullary myeloblastoma, granulocytic sarcoma, chloroma) is an extramedullary isolated malignant tumor of myeloblasts and immature myelocytes [1]. It can occur anywhere in the body affecting only one or sometimes more than two different organs (skin, gastrointestinal system, lymph nodes, central nervous system, heart) [2–4]. The myeloid sarcoma (MS) is often accompanied (as a relapse) or followed by acute myeloid leukemia (AML). It can also precede other blood or bone marrow disease [5]. According to the 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia, the MS is classified in the group of myeloid neoplasms and acute leukemia, subgroup of acute myeloid leukemia and related neoplasms [5].
MS occurs in patients with AML in 2–8% of cases [6]. Due to its low incidence, only small series and case reports are published. The age of the affected patients is variable due to the reported cases.
The clinical presentation of the extramedullary isolated tumor is dependent of its localization. The dominating clinical signs are associated with compressive effects, localized pain and bleeding. Therefore, contrast enhanced computer tomography (CT) and magnetic resonance imaging (MRi) are mostly used for the initial diagnosis [7].
The final diagnosis of MS is established with immunohistochemistry and immunophe-notyping [8].
Since there is a lack of a consensus for the treatment of MS, the proposed treatment whether for extramedullary MS or the one that is concomitant with AML is the standard AML-type chemotherapy, hematopoietic stem-cell transplanation and radiotherapy [8]. There are also reports for targeted therapy with new drugs such as: monoclonal antibody therapy with Gemtuzumab ozogamicin, Nucleoside analogues, demethyaltion agents and others [6, 9, 10].
If left untreated, the extramedullary MS almost always progresses to AML. The prognosis for this condition is variable but similar to that of AML [11].
Case report
In May 2018, a 22 years old male patient presented to our emergency department of surgery with clinical signs of intestinal obstruction accompanied with abdominal pain and vomiting. On a physical examination the abdomen was slightly distended and with palpable tumor in the right medial abdominal quadrant. The blood analysis showed normal leukocyte count of 7.0x109/l with differential count for Lymphocytes 24.2%, Monocytes 13.3% and Neutrophils 62.5%. Platelets count was 447x109/l, hemoglobin level of 158.0x109/l and CRP level of 4.4 mg/l.
The plain radiograph showed signs of small bowel obstruction (Figure 1).
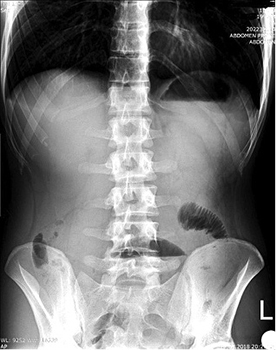 Figure 1
Figure 1
A contrast enhanced CT scan was indicated due to the palpable abdominal tumor and it revealed a formation that originates from the ileal mesentery involving the ileocolic artery and a part of the ileum with ileal wall thickening and partial obstruction (Figures 2, 3 and 4).
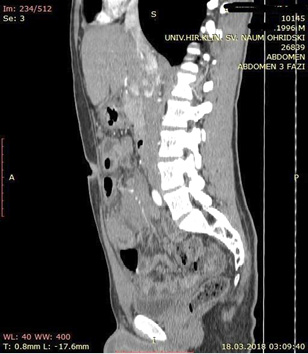 Figure 2
Figure 2 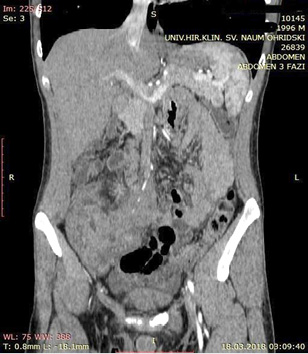 Figure 3
Figure 3
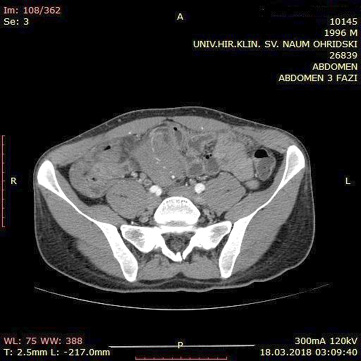 Figure 4
Figure 4
A surgical exploration was indicated the same day. During surgery, the tumor affected part of the mesentery that contains the ileocolic artery but with no vascular changes of the terminal ileum and the right colon. It was also affecting a part of the ileum wall which explained the small bowel obstruction (Figure 5, 6).
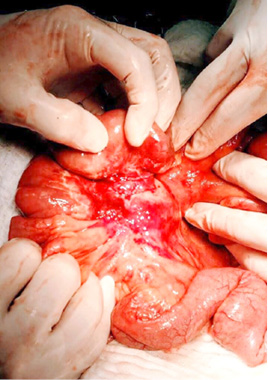 Figure 5
Figure 5 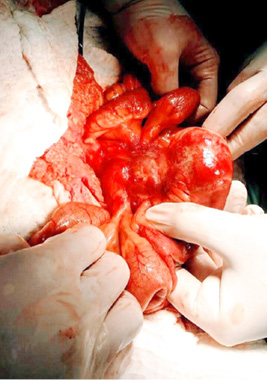 Figure 6
Figure 6
Due to the normal finding of the right colon, extirpation of the whole tumor was not attempted and only a resection of the affected ileum was undertaken with a primary anastomosis creation. The patient had uneventful postoperative period and was discharged on postoperative day 6.
The macroscopic pathology analysis revealed hemorrhagic elevations of the ileal mucosa with infiltrating lesion of the intestinal wall and luminal stenosis in up to 90%. The same was spreading in the mesenteric fat. Microscopic view showed 4–5 mitoses on large microscopic field (Figure 7).
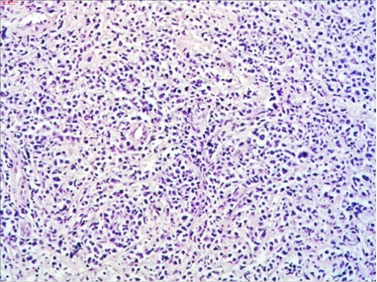
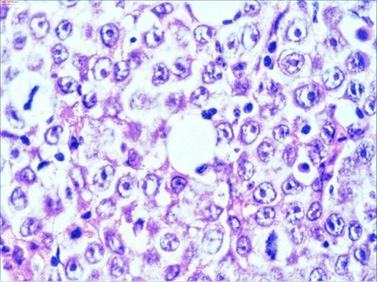
Figure 7. HE staining
The immunohistochemistry analysis found high expression for Lysozime, LCA, CD117 and CD68 and partial expression for CD11c and bcl–2 (Figure 8).
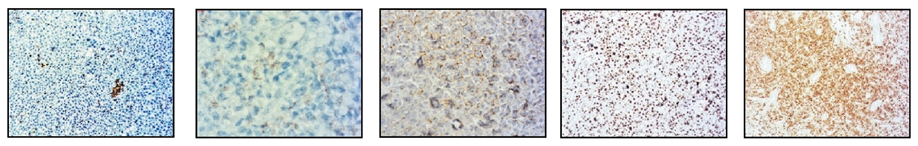
CD11 CD68 CD138 Ki67 CD117
Figure 8. Immunohistochemistry staining
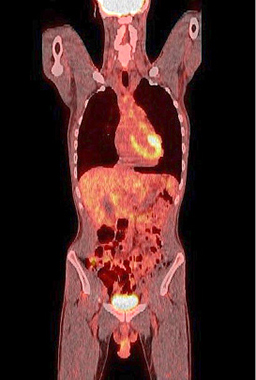
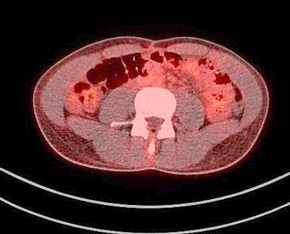
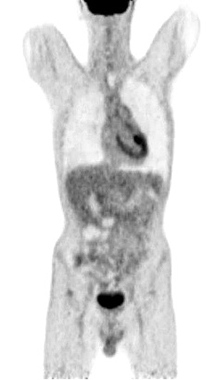
Figure 9. A normal finding of post-transplant PET/CT Scan
During the immunohistochemistry analysis, the patient was sent to the University Clinic for Hematology where a bone marrow biopsy was done. It showed no signs of leukemic process and the diagnosis of extramedullary myeloid sarcoma was established.
In the same time period (one month after the surgery), the patient developed AML and was admitted to the University Clinic for Hematology. A standard induction regimen therapy (7+3) was conducted and afterwards allogenic bone marrow transplant was performed at the Clinic for Hematology. The recovery period were uneventful.
One year after the operation, a PET/ CT scan performed showed normal distribution of F-18 FDG and no signs of residual intraabdominal tumor (Figure 9). In the present period, the patient is disease free.
Discussion
The extramedullary myeloid sarcoma with primary localization in the gastrointestinal system is rare and accounts with 6.5% of cases where the ileum is the most common site affected [12, 13]. The literature review reveals mostly single case reports [14–24]. Establishing its diagnosis is difficult. An isolated MS that can be misdiagnosed initially in up to 47% with some other hematologic malignancies, mostly malignant lymphoma [25]. The presence of MS with normal bone marrow finding has been reported in 30% of cases [26]. It certainly contributes for the diagnosis delay and postpones the beginning of the appropriate therapy. In our case the patient’s condition progressed to AML within a month after the surgery, although a previously done bone marrow biopsy was with normal finding.
The treatment for isolated MS or the one concomitant with AML includes a standard AML-type chemotherapy, since a higher rate of progression to AML in patients with isolated MS that have been treated with local excision only are reported [25, 27, 28].
Radiotherapy has been added in cases of MS previously treated with chemotherapy. Unfortunately no significant effect was found in matter of prolonged survival compared to those that were not radiated [8, 27, 29].
A few retrospective reports show the role of hematopoietic stem cell transplantation. Allogenic and autologous stem cell transplantation (SCT) was used in small groups of patients. In all the series, the groups of patients with any type of SCT reported significantly prolonged overall survival compared to those from non-SCT groups [6, 8].
Targeted therapy in its inception has been reported with various drugs such as Gemtuzumab ozogamicin, Imatinib mesylat, nucleoside analogues and demethyaltion agents [6, 9, 10, 30]. The reports are somewhere promising. Probably a model for identification of those patients that could have benefit is needed.
Conclusion
MS is related with uncertain prognosis and outcome. It is important to report and publish all newly diagnosed cases even as a case reports. It will lead, at least partially, to comprehend the clinical patterns of the extramedullary myeloid sarcoma. This could probably allow timely diagnosis and more efficient therapy for longer disease-free period and longer overall survival.
References
1. Brunning RD, Matutes E, Flandrin G, Vardiman J, Bennett J, Head D, Harris NL. Tumours of haematopoietic and lymphoid tissues. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification. Lyon, France: IARC Press, 2001, p. 104–105.
2. Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma 2006; 47(12): 2527–2541.
3. Antic D, Verstovsek S, Elezovic I, Grujicic D, Gotic M, Bila J, Perunicic M, Jakovic L. Spinal epidural granulocytic sarcoma in non-leukemic patient. Int J Hematol 2009; 89(1): 95–97.
4. Antic D, Vuckovic M, Elezovic I. Right atrial myeloid sarcoma causing superior vena cava syndrome. Br J Haematol 2008; 141(2): 134.
5. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114(5): 937–951.
6. Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D, Bisceglia M, Ponzoni M, Gentile A, Rinaldi P, Franco V, Vincelli D, Pileri AJr, Gasbarra R, Falini B, Zinzani PL, Baccarani M. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007; 21(2): 340–350.
7. Pui MH, Fletcher BD, Langston JW. Granulocytic sarcoma in childhood leukemia: imaging features. Radiology 1994; 190(3): 698–702.
8. Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011; 2(5): 309–316.
9. Piccaluga PP, Martinelli G, Rondoni M, Malagola M, Gaitani S, Isidori A, Bonini A, Gugliotta L, Luppi M, Morselli M, Sparaventi G, Visani G, Baccarani M. Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas. Leuk Lymphoma 2004; 45(9): 1791–1795.
10. Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol 2011; 29(5): 487–494.
11. Ghafoor T, Zaidi A, Al Nassir I. Granulocytic Sarcoma of the Small Intestine: An Unusual Presentation of Acute
Myelogenous Leukaemia. J Pak Med Assoc. 2010; 60(2): 133–135.
12. Kohl SK, Aoun P. Granulocytic Sarcoma of the Small Intestine. Arch Path Lab Med 2006; 130(10): 1570–1574.
13. Corpechot C, Lémann M, Brocheriou I, Mariette X, Bonnet J, Daniel MT, Bertheau P, Lavergne A, Modigliani R. Granulocytic sarcoma of the jejunum: a rare cause of small bowel obstruction. Am J Gastroenterol. 1998; 93(12): 2586–2588.
14. Wang P, Li Q, Zhang L, Ji H, Zhang CZ, Wang B. A myeloid sarcoma involving the small intestine, kidneys, mesentery, and mesenteric lymph nodes: A case report and literature review. Medicine 2017; 96(42): e7934.
15. He T, Guo Y, Wang C, Yan J, Zhang M, Xu W, He X, Zheng S. A primary myeloid sarcoma involving the small intestine and mesentery: case report and literature review. Int J Clin Exp Pathol. 2018; 11(8): 4158–4162.
16. Yoldaş T, Erol V, Demir B, Hoşcoşkun C. A rare cause of mechanical obstruction: Intestinal myeloid sarcoma. Ulus Cerrahi Derg. 2013; 30(3): 176–178.
17. Van de Voorde N, Min W, Salgado R. De Novo Myeloid Sarcoma as a Cause of Small Bowel Obstruction: A Case Report. J Belg Soc Radiol. 2017; 101(1): 28.
18. Aslan B, Tüney D, Erçetin Y, Bozkurt SU, Uprak TK. De novo myeloid sarcoma as a rare cause of small bowel obstruction: CT findings and histopathologic correlation. Radiol Case Rep. 2019; 14(12): 1487–1490.
19. Mizumoto R, Tsujie M, Wakasa T, Kitani K, Manabe H, Fukuda S, Okada K, Satoi S, Ishikawa H, Kawasaki T, Hanamoto H, Yukawa M, Inoue M. Isolated myeloid sarcoma presenting with small bowel obstruction: A case report. Surg Case Rep. 2020; 6(1): 2.
20. Kim NR, Lee WK, Lee JI, Cho HY. Multiple Jejunal Myeloid Sarcomas Presenting with Intestinal Obstruction in a Non-leukemic Patient: A Case Report with Ultrastructural Observations. Korean J Pathol. 2012; 46(6): 590–594.
21. Yoshida M, Ogami T, Morgenstern N, Du L. Myeloid sarcoma resulting in a small bowel obstruction with multiple site involvement including ileum and appendix. J Surg Case Rep. 2019; 2019(9): rjz248.
22. Cicilet S, Tom FK, Philip B, Biswas A. Primary myeloid sarcoma of small bowel. BMJ Case Rep. 2017.
23. Girelli CM, Carsenzuola V, Latargia M, Aguzzi A, Serio G. Small-bowel myeloid sarcoma: Report of a case with atypical presentation. Int J Surg Case Rep. 2014; 5(9): 613–616.
24. Mandal PK, Dolai TK. A rare case of isolated myeloid sarcoma of the small gut with inv(16) (p13;q22) without bone marrow involvement. Blood Res. 2014; 49(1): 66–69.
25. Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer 2002; 94(6): 1739–1746.
26. Jung SH, Kim HC, Yu CS, Kim JC. Solitary preleukemic granulocytic sarcoma as a cause of small bowel obstruction. Gut Liver 2007; 1(1): 82–86.
27. Lan TY, Lin DT, Tien HF, Yang RS, Chen CY, Wu K. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009; 122(4): 238–246.
28. Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, Thomas DA, Garcia-Manero G, Ferrajoli A, Manning JT, Keating MJ, Albitar M, O’Brien S, Giles FJ. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia 2003; 17(6): 1100–1103.
29. Dusenbery KE, Howells WB, Arthur DC, Alonzo T, Lee JW, Kobrinsky N, Barnard DR, Wells RJ, Buckley JD, Lange BJ, Woods WG. Extramedullary leukemia in children with newly diagnosed acute myeloid leukemia: A report from the Children’s Cancer Group. J Pediatr Hematol Oncol. 2003; 25(10): 760–768.
30. Védy D, Muehlematter D, Rausch T, Stalder M, Jotterand M, Spertini O. Acute myeloid leukemia with myeloid sarcoma and eosinophilia: prolonged remission and molecular response to imatinib. J Clin Oncol 2010; 28(3): e33–e35.