Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2022, vol. 21(3–4), pp. 202–207 DOI: https://doi.org/10.15388/LietChirur.2022.21.66
Prognosis of Stage IV Gastric Cancer Patients
Ho Gun Kim
Division of Gastroenterologic Surgery, Department of Surgery, Chonnam National University Medical School, Gwangju, Korea
E-mail: dr4477@hanmail.net
Dong Yeon Kang
Division of Gastroenterologic Surgery, Department of Surgery, Chonnam National University Medical School, Gwangju, Korea
E-mail: 71717711@hanmail.net
Jae Hyuk Lee
Department of Pathology, Chonnam National University Medical School, Gwangju, Korea
E-mail: jhlee@jnu.ac.kr
Dong Yi Kim
Division of Gastroenterologic Surgery, Department of Surgery, Chonnam National University Medical School, Gwangju, Korea
E-mail: dockim@jnu.ac.kr
Abstract. Aim. This study evaluated the survival of gastric cancer patients with metastasis to the hepatoduodenal, retropancreatic, mesenteric, and para-aortic lymph nodes. Materials and methods. We analyzed the survival rate of 435 gastric cancer patients who underwent operation from 2001 to 2010 at the Department of Surgery, Chonnam National University Hospital. There were 43, 25, 16, and 55 patients with metastasis to the hepatoduodenal, retropancreatic, mesenteric, and para-aortic nodes, respectively. Results. Based on tumor location, metastasis to the para-aortic lymph nodes was more common in upper-third cancer, and that to the hepatoduodenal lymph nodes was more common in lower-third cancer. The survival rate of patients with non-regional lymph node metastasis was better than that of patients with hepatic metastasis or peritoneal dissemination (p < 0.05). Conclusion. We recommend performing a more extended lymphadenectomy than a D2 lymphadenectomy in patients with advanced gastric cancer those having metastasis to the hepatoduodenal nodes.
Key words: prognosis, stomach neoplasm, lymphadenectomy.
Received: 2022/08/10. Accepted: 2022/09/15.
Copyright © 2022 Ho Gun Kim, Dong Yeon Gang, Jae Hyuk Lee, Dong Yi Kim. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Although the incidence of gastric cancer is declining, it is still one of the leading causes of death by malignant tumors worldwide. The prognosis for stage IV gastric cancer patients is very poor, even after surgical treatment [1, 2].
In Korea and Japan, gastrectomy with extended lymph node dissection, which involves the dissection of more nodes than those invaded by the tumor, has recently become the standard surgery for advanced gastric cancer. However, Western groups have found no survival benefit of extended lymphadenectomy as compared to limited lymphadenectomy for patients with advanced gastric cancer [3, 4].
This study evaluated the survival of patients with metastasis to the hepatoduodenal, retropancreatic, mesenteric, and para-aortic lymph nodes and compared it with that of other distant metastases, such as hepatic metastasis and peritoneal dissemination, to determine the extent of lymph node dissection required in advanced gastric cancer patients.
Materials and methods
A total of 435 node-positive or gastric cancer patients with distant metastasis (305 males and 130 females; age range: 17 to 85 years; mean: 56.3 years), who underwent gastric resection with D2 or more extended lymph node dissection over a 15-year period from 2001 to 2010, were enrolled in this study.
There were 43, 25, 16, and 55 patients with metastasis to the hepatoduodenal, retropancreatic, mesenteric, and para-aortic lymph nodes, respectively. There were 69, 202, and 25 patients with hepatic or peritoneal metastasis, or both, respectively.
The data were analyzed using the chi-squared test and the unpaired Student’s t-test. The overall survival rates were calculated using the Kaplan-Meier method. We compared the survival curves of the patients using the Cox regression method. A P value <0.05 was considered statistically significant.
Results
Hepatic metastasis was more common in well- or moderately differentiated cancer, while peritoneal dissemination was more common in poorly differentiated cancer (P < 0.001) (Fig. 1). According to tumor location, metastasis to the para-aortic lymph nodes was more common in cancer of the upper third of the stomach, while that to the hepatoduodenal lymph nodes was more common in lower-third cancer. Peritoneal dissemination was more common in patients with cancer involving the entire stomach (Fig. 2).

Figure 1. Pattern of metastasis according to histologic type (hepatic metastasis was more common in well- or moderately differentiated cancer and peritoneal dissemination was more common in poorly differentiated cancer (P < 0.001)
The 5-year survival rate of patients with metastasis to the hepatoduodenal, retropancreatic, mesenteric, and para-aortic lymph nodes was 43.7, 13.0, 21.0, and 28.2%, respectively. The survival of patients with non-regional lymph node involvement, such as hepatoduodenal, mesenteric, and para-aortic lymph nodes, was better than that of patients with hepatic metastasis or peritoneal dissemination (Fig. 3). The median survival of those patients was 2.39±0.96, 1.68±0.40, 2.19±0.45, and 1.41±0.19 years, respectively (Table 1).
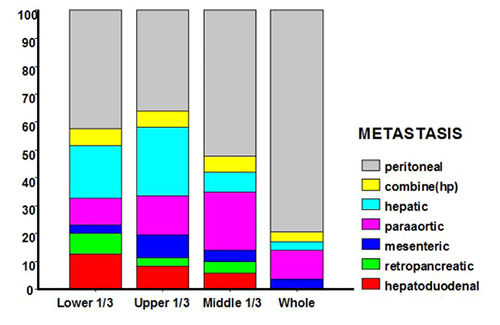
Figure 2. Pattern of metastasis according to tumor location (based on the gastric cancer location, metastasis to the para-aortic lymph nodes was more common in upper-third cancer, and that to hepatoduodenal lymph nodes was more common in lower-third cancer; peritoneal dissemination was more common in patients with cancer involving the entire stomach)
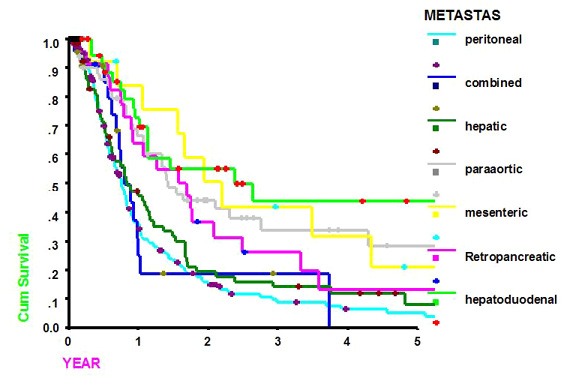
Figure 3. Survival curves according to the pattern of metastasis (the survival of patients with non-regional lymph node metastasis, such as hepatoduodenal, mesenteric, and para-aortic lymph nodes, was better than that of patients with hepatic metastasis or peritoneal dissemination)
Table 1. Five year survival time according to the patterns of metastasis
|
Metastasis (n) |
5-year survival rate (%) |
Median survival time (year) |
|
Hepatoduodenal (43) |
43.7 |
2.39±0.96*** |
|
Retropancreatic (25) |
13.0 |
1.68±0.40* |
|
Mesenteric (16) |
21.0 |
2.19±0.45** |
|
Paraaortic (55) |
28.2 |
1.41±0.19*** |
|
Hepatic (69) |
12.0 |
0.83±0.16 |
|
Peritoneal (202) |
5.2 |
0.75±0.05 |
|
Combined (H+P) (25) |
0 |
0.80±0.14 |
*P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Depth of invasion of the gastric wall (T) is correlated with reduced survival, while the regional lymphatic spread is probably the most powerful prognostic factor for gastric cancer [5]. The International Union Against Cancer (UICC) TNM staging system defined a new system for classifying gastric cancer, based on the number of metastatic nodes. In this system, involvement of the hepatoduodenal, retropancreatic, mesenteric, and para-aortic lymph nodes is classified as distant metastasis [6].
The role of extended lymphadenectomy in the surgical management of advanced gastric cancer is controversial. Although many studies report the value of extended lymphadenectomy in advanced gastric cancer, both the Dutch [5] and Medical Research Council trials [7] did not demonstrate any survival benefit of extended lymphadenectomy. Furthermore, both trials revealed increased operative morbidity and mortality after extended lymphadenectomy.
Curative resection is the treatment of choice for gastric cancer, but it is unclear whether this operation should include an extended lymphadenectomy. Currently, gastrectomy with extended lymphadenectomy is the standard operative procedure for gastric cancer in Korea and Japan [8, 9], while limited lymph node dissection is the standard procedure in Western countries [5, 10].
Western groups have not seen the same survival benefits as Eastern groups [3, 4], although there are many encouraging results. Roukos et al. [11] reported that D2 dissection has a survival benefit for patients with lymph node metastasis. Yildirim et al. [12] reported a survival benefit for D2 lymph node dissection in patients with muscularis propria gastric cancer or in patients in which the tumor penetrated the serosal layer. Ramacciato et al. [13] reported an improved survival rate after extended lymph node dissection in Stage II and III gastric cancer, particularly in patients with N1 and N2 node metastasis. Several Japanese studies also showed an increased 5-year survival rate when an extended lymphadenectomy was performed. Previously, we showed that the survival rate after D2 lymph node dissection is significantly better than that after D1 lymph node dissection in patients with early or advanced gastric cancer.
Many investigators have reported that aggressive surgery, such as extended lymph node dissection (D2 or more) or gastrectomy with pancreatectomy [5, 7, 14], increases operative morbidity and mortality. The Dutch trial [5] did recommended a routine D2 lymph node dissection because of the high morbidity (43%) and mortality (10%) and the lack of a difference in the 5-year survival rates when comparing D1 dissection with D2 lymph node dissection [4, 5, 7]. By contrast, Günther et al. [15] did not see an increase in the overall postoperative complications or death rate after extended lymph node dissection and recommended extended lymph node dissection in patients with advanced gastric cancer. Roviello et al. [16] also reported that extended lymph node dissection had low postoperative complications and mortality rates and increased the probability of long-term survival, even in patients with regional lymph node involvement. Some investigators reported that highly selected acceptable risk surgical candidates with stage IV gastric cancer should be considered for management with surgical resection and might be associated with a survival advantage in subgroups of patients with metastatic gastric cancer [17, 18].
Lymphadenectomy, which is a prognostic factor that can be influenced by the surgeon [19], improves the survival rate in gastric cancer, although no extensive prospective randomized trial has examined this. Viste et al. [20] reported that the survival of patients who underwent extensive lymph node dissection exceeded that of patients not undergoing dissection. Furthermore, Ichiyoshi et al. [21] attributed lymph node recurrence to inadequate lymph node dissection. The Japanese guidelines recommend extended radical gastrectomy with lymphadenectomy for patients with T1-3, N3 or T4, N2-3 lesions without M1 [22]. Kasakura et al. [23] recommended that metastatic lymph nodes be resected as far as possible and recommended D2 dissection for T1, N1 or T2, N1 gastric cancer. We examined the effect of D2 lymph node dissection on the survival benefit in patients who had undergone surgery for gastric cancer. The survival of gastric carcinoma patients with hepatoduodenal lymph node metastasis was good.
Conclusion
We recommend performing a more extended lymph node dissection than D2 lymph node dissection in patients with advanced gastric cancer, especially in those with suspected metastasis to the hepatoduodenal lymph nodes.
References
1. Yagi Y, Seshimo A, Kameoka S. Prognostic factors in stage IV gastric cancer: univariate and multivariate analyses. Gastric Cancer 2000; 3: 71–80.
2. Sougioultzis S, Syrios J, Xynos ID, Bovaretos N, Kosmas C, Sarantonis J, Dokou A, Tzivras D, Zografos G, Felekouras E, Papalambros E, Tsavaris N. Palliative gastrectomy and other factors affecting overall survival in stage IV gastric adenocarcinoma patients receiving chemotherapy: a retrospective analysis. Eur J Surg Oncol 2011; 37: 312–318.
3. Smith JW, Shiu MH, Kelsey L, Brennan MF. Morbidity of radical lymphadenectomy in the curative resection of gastric carcinoma. Arch Surg 1991; 126: 1469–1473.
4. Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomized comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995; 345: 745–748.
5. Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H. Extended lymph-node dissection for gastric cancer. Dutch Gastric Cancer Group. N Engl J Med 1999; 25: 908–914.
6. Sobin LH, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumours, 6th edition. New York: Wiley, 2002.
7. Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trials. Br J Cancer 1999; 79: 1522–1530.
8. Soga J, Ohyama S, Miyashita K, Suzuki T, Nashimoto A, Tanaka O, Sasaki K, Muto T. A statistical evaluation of advancement of lymphadenectomy for cure. World J Surg 1988; 12: 398–405.
9. Kim HG, Ghu HD, Yun SK, Ryu SY, Kim DY. Clinicopathological features of female gastric carcinoma patients with curative resection: comparison with male patients. Chonnam Med J 2012; 48: 86–90.
10. Heberer G, Teichmann RK, Kramling JH, Günther B. Results of gastric resection for carcinoma of the stomach. The European experience. World J Surg 1988; 12: 374–381.
11. Roukos DH, Lorenz M, Encke A. Evidence of survival benefit of extended (D2) lymphadenectomy in Western patients with gastric cancer based on a new concept: a prospective long-term follow-up study. Surgery 1998; 123: 573–578.
12. Yildirim E, Celen O, Berber O, Glu N. The Turkish experience with curative gastrectomies for gastric carcinoma: is D2 dissection worthwhile? J Am Coll Surg 2001; 192: 25–37.
13. Ramacciato G, Aurello P, D’Angelo F, Cicchini C, Sternberg CN. Does extended lymphadenectomy influence prognosis of gastric carcinoma after curative resection? Hepatogastroenterology 2000; 47: 1470–1474.
14. Martin RC, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg 2002; 236: 159–165.
15. Günther K, Horbach T, Merkel S, Meyer M, Schnell U, Klein P, Hohenberger W. D3 lymph node dissection in gastric cancer: evaluation of postoperative mortality and complications. Surg Today 2000; 30: 700–705.
16. Roviello F, Marrelli D, Morgagni P, de Manzoni G, Di Leo A, Vindigni C, Saragoni L, Tomezzoli A, Kurihara H; Italian Research Group for Gastric Cancer. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol 2002; 9: 894–900.
17. Lim S, Muhs BE, Marcus SG, Newman E, Berman RS, Hiotis SP. Results following resection for stage IV gastric cancer; are better outcomes observed in selected patient subgroups? J Surg Oncol 2007; 95: 118–122.
18. Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Surgical resection of stage IV gastric cancer and prognosis. Anticancer Res 2007; 27: 4381–4386.
19. Marubini E, Bozzetti F, Miceli R, Bonfanti G, Gennari L; Gastrointestinal Tumor Study Group. Lymphadenectomy in gastric cancer: prognostic role and therapeutic implications. Eur J Surg Oncol 2002; 28: 406–412.
20. Viste A, Svanes K, Janssen CW, Maartmann-Moe H, Søreide O. Prognostic importance of radical lymphadenectomy in curative resections for gastric cancer. Eur J Surg 1994; 160: 497–502.
21. Ichiyoshi Y, Toda T, Minamisono Y, Nagasaki S, Yakeishi Y, Sugimachi K. Recurrence in early gastric cancer. Surgery 1990; 107: 489–495.
22. Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer 2002; 5: 1–5.
23. Kasakura Y, Mochizuki F, Wakabayashi K, Kochi M, Fujii M, Takayama T. An evaluation of the effectiveness of extended lymph node dissection in patients with gastric cancer: a retrospective study of 1403 cases at a single institution. J Surg Res 2002; 103: 252–259.