Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2023, vol. 22(2), pp. 99–105 DOI: https://doi.org/10.15388/LietChirur.2023.22.84
Pancreatic Mucinous Cystic Neoplasm with Associated Invasive Carcinoma: A Case Report and Literature Review
Kristina Marcinkeviciute
Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
E-mail: kristinamarcinkeviciute99@gmail.com
Digne Jurkeviciute
Consultative Polyclinic Department, National Cancer Institute, Vilnius, Lithuania
E-mail: digne.j@gmail.com
Rokas Stulpinas
National Center of Pathology, Affiliate of Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania
E-mail: rokas.stulpinas@vpc.lt
Eugenijus Stratilatovas
Department of Abdominal and General Surgery and Oncology, National Cancer Institute, Vilnius, Lithuania
E-mail: eugenijus.stratilatovas@nvi.lt
Audrius Dulskas
Department of Abdominal and General Surgery and Oncology, National Cancer Institute, Vilnius, Lithuania
E-mail: audrius.dulskas@gmail.com, audrius.dulskas@nvi.lt
ORCID: https://orcid.org/0000-0003-3692-8962
Abstract. Background. Pancreatic mucinous cystic neoplasm (PMCN) with associated invasive carcinoma is a rare entity. According to the World Health Organisation (WHO) 2010, PMCN with associated invasive carcinoma is referred to the malignant lesions of the pancreatic epithelial tumour. Case report. A 52-year-old female patient presented with pain in the umbilical and epigastric regions for 5 months and noticed a solid visible tumour on the left side of the abdomen 3 months ago when she lied down. The level of the CA125 was 47.64 U/ml (normal value <35 U/ml). Abdominal and pelvic magnetic resonance imaging (MRI) showed a cystic multiseptal mass in the left iliac region, defined as a left ovary tumour, while Computed tomography scan revealed a cystic tumour of the pancreatic tail. The patient underwent a resection of the pancreatic tail with a 20 cm cystic solid tumour, splenectomy and left hemicolectomy. Histopathology report confirmed mucinous cystic neoplasm of the pancreatic tail with associated invasive carcinoma (combined badly differentiated (G3) ductal (40%) and undifferentiated (G4) anaplastic (60%) carcinoma) pT1bN0. Postoperative course complicated with wound infection. The patient was discharged on postoperative day 10. The patient is still alive 2 years on follow-up. Conclusions. PMCN with associated invasive carcinomas are rare lesions of pancreas with relatively benign course. This malignant pancreatic tumour displays morphologies as pleomorphic epithelial cells and relatively mononuclear spindle cells, and not always tends to have underlying ovarian type stroma. The comprehensive histopathological examination of the tumour is necessary in order to cure most MCN patients with minimally invasive types.
Key words: pancreatic mucinous cystic neoplasm, IPMN, invasive carcinoma of the pancreas, pancreatic lesions, pancreatic cancer, mucinous cystic neoplasms, case report.
Received: 2023/02/20. Accepted: 2023/03/31.
Copyright © 2023 Kristina Marcinkeviciute, Digne Jurkeviciute, Rokas Stulpinas, Eugenijus Stratilatovas, Audrius Dulskas. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Pancreatic mucinous cystic neoplasm (PMCN) is a rare mucin producing cystic neoplasm that is histologically described by ovarian-type stroma [1]. This tumour is usually solitary and does not have a connection to the pancreatic ductal system. The incidence of PMCN is about 8% of all resected cystic lesions of the pancreas [2]. They are slow-growing, occur in the pancreatic duct epithelium, and predominantly affect women [3]. The PMCN is defined as mucinous cystic neoplasm (MCN) with an invasive carcinoma if the tumour has a contact with the pancreatic duct, or the tumour has mural nodularity and appears to be larger than 3 cm [4]. The prevalence of this form of tumour ranges from 6 to 55% of all mucinous cystic neoplasms [5]. Approximately 150 such cases have been reported in the literature so far [4]. Moreover, in our case the tumour is large in size with no underlying ovarian type stroma and rare TP53 mutation is present. Usually, this neoplasm is found in the body or tail of the pancreas of the middle-aged (perimenopausal) women (more than 98% of all cases) and rarely of the older man [4, 6]. The malignant form is associated with the age. It is more frequently found in older patients due to increased possibility of adenoma progression to carcinoma as the individual ages [4].
Here we present a case of PMCN with associated invasive combined G3 ductal and G4 anaplastic carcinoma (IDC), with no underlying ovarian type stroma.
Case description
The patient has given their consent for the report. Approval from the Institutional Review Board was granted.
A 52-year-old female patient who complained about the pain in the umbilical and epigastric regions for 5 months presented to our clinic. Three months ago, she noticed a solid tumour on the left side of the abdomen when lying down.
The level of the CA125 was slightly increased – 47.64 U/ml (normal value <35 U/ml). Abdominal and pelvic magnetic resonance imaging (MRI) showed a cystic multiseptal mass in the left iliac region, defined as a left ovary tumour, while computed tomography (CT) scan of thorax, abdomen and pelvis revealed a cystic tumour of the pancreatic tail, cT3N0M0 – possibly a cystadenoma or cystadenocarcinoma.
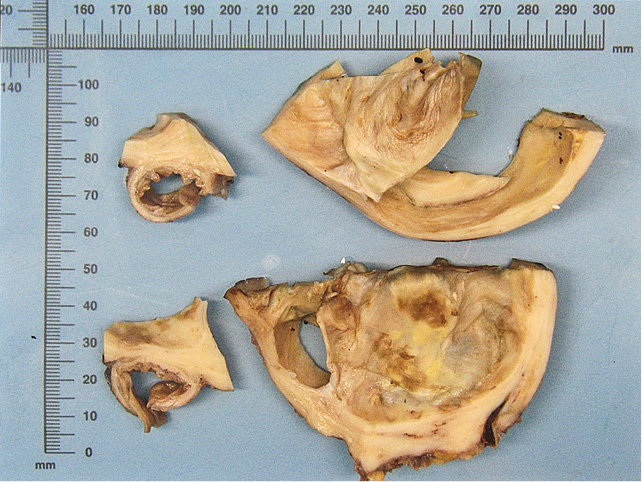
Figure 1. Multilocular cystic lesion with smooth inner surface, thick fleshy capsule and minimal haemorrhagic residue
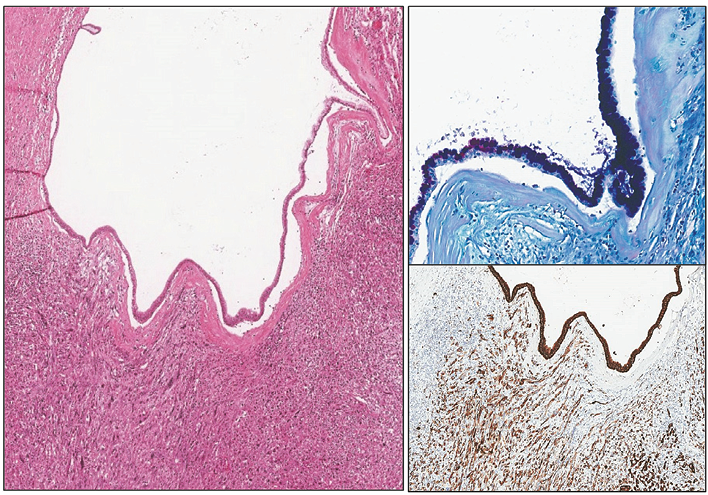
Figure 2. At low power, a cyst lined by mucinous (AB/PAS on top right) epithelium overlying invasive component composed of pleomorphic spindle cells with strong pan-cytokeratin reaction (PanCK on bottom right)
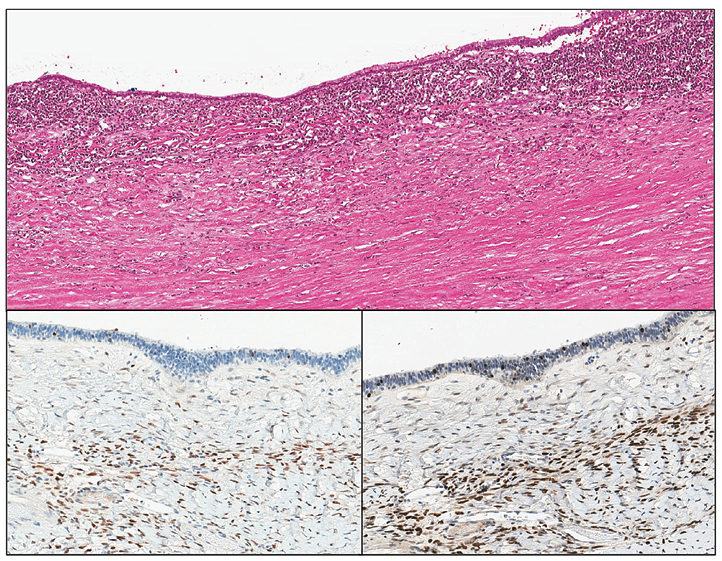
Figure 3. The ovarian type stroma of larger MCNs is often hyalinized and hypocellular, but stromal estrogen (bottom left) and progesterone receptors (bottom right) are present
The patient underwent a resection of the pancreatic tail with a 20 cm tumour, splenectomy and left hemicolectomy. Histopathology showed mucinous cystic neoplasm of the pancreatic tail with associated invasive carcinoma (combined poorly differentiated (G3) ductal (40%) and undifferentiated (G4) anaplastic (60%) carcinoma) (Figure 1). It was staged as pT1bN0 considering that the maximum size of the identified invasive carcinoma was 8 mm and the 23 found regional lymph nodes were not involved (did not exceeded 8 mm). The intravascular invasion was not identified. The resection was R0. Microscopically pancreatic lesion was composed of a thick, hyalinized capsule surrounding mucin filled cystic cavity with peripheral septation and smaller secondary spaces. Inner surface of the cyst was lined with monomorphic PAS+ or AB+/PAS+ mucinous epithelium, focally exhibiting intraluminal micropapillarisation, nucleomegaly and stratification. An invasive component consisted of either irregular glandular structures (“ductal”) or solid sheets of eosinophilic pleomorphic spindle cells (“anaplastic”) was noted in a few slides (Figure 2). Invasive carcinoma had INI1 expression retained loss of SMAD4, strong diffuse nuclear positivity for p53 and was negative for Inhibin and CK5. The ovarian type stroma was not prominent (as it often is the case in large MCNs) but nuclear ER or PR positivity was unequivocal (Figure 3). Next-generation sequencing (NGS) identified PTPN11 and TP53 mutations.
Postoperative course complicated with wound infection. The antibiotics were administered according to bacteria culture test and the passive drainage with Penrose drains was applied. The patient was discharged on the 10th day after the operation. No further treatment was needed. She is still alive without a progression 27 months following the surgery (Figure 4).
|
|
|||
|
2019-10 |
2020-02 |
2020-04 |
2022-07 |
|
Early signs of symptoms |
PMCN with associated |
Resection of the pancreatic |
Patient alive without |
Figure 4. Timeline of the presented case
Discussion
Mucinous cystic neoplasm might progress to invasive form of the tumour. It occurs in up to a third of all cases (the frequency differs and depends on the study) and those are named as MCN with associated invasive carcinoma [4, 7]. There have only been about 150 such patients described in the literature but the number of patients with TP53 mutation and with no underlying ovarian type stroma (as in this case) is significantly lower [4]. IPMNs have a wide range of atypical grades, from low-grade dysplasia to aggressive malignancy [8, 9]. The likelihood of finding even small-sized IPMNs has increased due to recent advancements in radiologic imaging [10]. PMC’s aetiology and pathophysiology are poorly understood. It is only clearly known that the mucin-producing cystic neoplasm histologically usually features the epithelium underlaid with ovarian-type stroma and is a precursor of the pancreatic ductal carcinoma [11]. However, a medullary phenotype was substantially related to SI, a wild-type KRAS gene, and a family history of any cancer in a first-degree relative in the study of Wilentz et al. [12]. Kryklyva et al described the first PMC case linked to a POLE mutation [13].
Patients with MCN can complain about abdominal pain. It is reported that for some patients it might start even in the right iliac fossa and radiate into the right lumbar region, while most of these patients admits left-sided, intermittent abdominal pain [14, 15]. Some patients can present with acute pancreatitis that could possibly result from pancreatic duct compression [16]. Some people with such diagnosis come to the hospital due to the lump in the left part of their abdomen [15, 17]. About quarter of the patients might appear asymptomatic, and the tumour might be detected at a regular health check-up [16, 18, 19]. In our case, the patient reported pain in the umbilical and epigastric regions and a solid visible tumour on the left side of the abdomen.
Laboratory tests are non-informative and normal range of cancer markers could not deny the diagnosis of pancreas cancer, therefore the main role in diagnostics takes visual investigations. In some reported cases an ultrasound-guided aspiration of the cystic fluid shows a normal [19] while in others a high level of CEA and CA19-9 [17]. However, CA19-9, the tumour marker of the pancreatic cancer, tends to be higher in invasive form compared to non-invasive form of the MCN [4]. Diagnostic tests mainly include abdominal and/or pelvic MRI and/or CT scan for visualisation of the tumour. MCN with invasive carcinoma should be at least 3 cm size, with the thicker cyst walls and while MCN is usually well circumscribed, malignant form is characterized by enhancing mural nodularity and calcification in the wall [4, 7]. The change of the pancreatic duct diameter with distal part of the pancreas atrophy is another feature found in an invasive form of the tumour. MRI tends to be more specific for interpreting internal components of this mass. In the majority of the reported cases, abdomen or/and pelvic CT was performed first followed by a more detailed diagnosis using the MRI scan [15, 18, 19]. Endoscopic ultrasound (EUS) can also be used for diagnostic as it better estimates the lesions and there is a possibility to take some fluid or cystic mural mass for histopathological investigation during the EUS [7]. Furthermore, positron emission tomography (PET) scan in some studies showed to be an accurate diagnostic test for differentiation of benign and malignant forms because it displayed hypermetabolic activity at the periphery of the mass [7, 15]. In addition to malignant IPMN, small-sized concurrent PDAC may also be detected using the ERCP with cytology [20]. The selection criteria for ERCP with cytology indication, however, are still not established. Therefore, routine ERCP for fluid or brush sampling in patients with IPMNs is not advised and should only be performed in research context [21]. In our case MRI and CT scan both were informative and none of the other visual investigations were performed. In most cases, the tumour is in the body and tail of the pancreas, while head of the pancreas remains unusual place for it to appear [22]. In this paper, the whole body CT scan revealed a cystic tumour of the pancreatic tail. At the first glance during the visual investigation, the malignant form of the tumour might be suspected because of the greater size of it. This piece of research suggests that MCN with and without an associated invasive carcinoma size significantly differs as 5.4 and 9.4 cm moderately [4]. In our case, the cystic mass was about 20 cm, so it could be expected to be malignant. The study [4] also refers that if intracystic papillary nodules are ≥1 cm they are highly associated with an invasive carcinoma.
In pathological investigation, MCN presents with ovarian-type stroma (OTS), which means spindle-shaped cells with scarce cytoplasm and round or elongated nucleus. If OTS is vague, then progesterone receptors (PR) immunohistochemical staining confirms MCN diagnosis. Its immunoreactivity is high and usually diffuses in low-grade dysplasia (LGD) or intermediate-grade dysplasia (IGD) but is weaker in high-grade dysplasia/carcinoma in situ (HGD/CIS). About 20% of all invasive forms might be a subset of undifferentiated/ sarcomatoid carcinoma and even have unusual osteoclast-like giant cells [4]. MCN with an invasive carcinoma has an expression of a high-molecular-weight transmembrane mucin (MUC1) that plays an important role in carcinogenesis and its positivity is related to poorer prognosis. Another feature that signs a worse prognosis is KRAS mutation in codon 12 or 13 [11]. This finding can also be identified in MCN, but the frequency of it might vary. For example, it is rarely reported in low-grade dysplasia of MCN [11]. In fact, a KRAS mutation is present in 60–80% of IPMN, as well as an overexpression of TP53 in 10–40% of high-grade IPMN and 40–60% of invasive carcinomas that have formed on IPMN [23]. During the histological examination, it is crucial to evaluate microscopic invasion and find out whether it is an early (T1a < 0.5 cm, T1b – 0.5 to 1 cm, T1c >1 cm) or advanced (T2 or higher grade > 2 cm) invasion [4, 11]. Hui et al showed that patients with MCN with an invasive T1a or T1b carcinoma should be under continuous monitoring instead of receiving aggressive therapy [24]. Moreover, if invasive carcinoma is detected histopathologically as multifocal, it could be a sign to malignant behaviour of the tumour even if it is a small invasive carcinoma. In our case, a combined poorly differentiated (G3) ductal (40%) and undifferentiated (G4) anaplastic (60%) carcinoma with early (T1b) invasion was detected, and NGS identified PTPN11 and TP53 mutations.
The prognosis of MCNs after the radical surgery is excellent with the probability of five-year survival of 84–93% [4, 16, 25]. The patients with MCN with an invasive carcinoma have lower survival rates – about 26–63% [4, 7]. Metastatic lymph nodes are related to poor prognosis. Moreover, if MCN invades other organs, it usually affects ovaries and colon [4]. It is also found in our case – the tumour was infiltrating mesocolon of transvers colon and the greater omentum. However, the majority of patients with MCN with minimally invasive forms is cured, especially if the neoplasm is well investigated histopathologically [26].
Conclusions
This piece of research confirmed that the mucinous cystic neoplasm of the pancreas with associated invasive carcinoma is a rare malignant pancreatic tumour, and typically displays several morphologies, including pleomorphic epithelial cells and relatively mononuclear spindle cells. However, most MCN patients with minimally invasive types are cured, particularly if the tumour is thoroughly examined histopathologically.
Acknowledgements. None.
Declaration of interest statement. The authors report there are no competing interests to declare.
Data availability statement. The data that support the findings of this study are available from the corresponding author, A. D., upon reasonable request.
References
1. Munekage M, Kohsaki T, Uemura S, Kitagawa H, Namikawa T, Hanazaki K. Mucinous cystadenocarcinoma of the pancreas with anaplastic carcinoma: A case report and review of the literature. Mol Clin Oncol 2016; 4(4): 483–486. DOI: 10.3892/mco.2016.743.
2. Din NU, Zubair M, Abdul-Ghafar J, Ahmad Z. Pancreatic mucinous cystic neoplasms: a clinicopathological study of 11 cases and detailed review of literature. Surgical and Experimental Pathology 2020; 3(1): 6. DOI: https://doi.org/10.1186/s42047-020-0059-2.
3. Eloubeidiv MA, Hawes RH. Mucinous Tumors of the Exocrine Pancreas. Cancer Control 2000; 5: 445–451. DOI: 10.1177/107327480000700507.
4. Jang KT, Park SM, Basturk O, Bagci P, Bandyopadhyay S, Stelow EB, Walters DM, Choi DW, Choi SH, Heo JS, Sarmiento JM, Reid MD, Adsay V. Clinicopathologic Characteristics of 29 Invasive Carcinomas Arising in 178 Pancreatic Mucinous Cystic Neoplasms With Ovarian-type Stroma. Am J Surg Pathol 2015; 39(2): 179–187. DOI: 10.1097/PAS.0000000000000357.
5. Naveed S, Qari H, Banday T, Altaf A, Para M. Mucinous Cystic Neoplasms of Pancreas. Gastroenterology Res 2014; 7(2): 44–50. DOI: 10.14740/gr600e.
6. Naganuma S, Honda K, Noriki S, Kimura S, Murakami M, Koneri K, Katayama K, Yamaguchi A, Itoh H. Ruptured mucinous cystic neoplasm with an associated invasive carcinoma of pancreatic head in a pregnant woman: report of a case and review of literature. Pathol Int 2011; 61(1): 28–33. DOI: 10.1111/j.1440-1827.2010.02609.x.
7. Fung C. Case 38: Mucinous Cystic Neoplasm in Pancreatic Head. Pancreatic Imaging 2017; 159–162. DOI: 10.1007/978-3-319-52680-5_38.
8. Basturk O, Hong SM, Wood LD. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol 2015; 39(12): 1730–1741. DOI: 10.1097/PAS.0000000000000533.
9. Adsay NV, Fukushima N, Furukawa T, et al. Intraductal neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Lyon: IARC, 2010, p. 304–313.
10. Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg 2004; 239(6): 788–799. DOI: 10.1097/01.sla.0000128306.90650.aa.
11. Sawai H, Kurimoto M, Koide S, Kiriyama Y, Haba S, Matsuo Y, Morimoto M, Koide H, Kamiya A, Yamao K. Invasive Ductal Carcinoma Arising in Mucinous Cystic Neoplasm of Pancreas: A Case Report. Am J Case Rep 2019; 20: 242–247. DOI: 10.12659/AJCR.914092.
12. Wilentz RE, Goggins M, Redston M, Marcus VA, Adsay NV, Sohn TA, Kadkol SS, Yeo CJ, Choti M, Zahurak M, Johnson K, Tascilar M, Offerhaus GJA, Hruban RH, Kern SE. Genetic, Immunohistochemical, and Clinical Features of Medullary Carcinoma of the Pancreas. Am J Pathol 2000; 156(5): 1641–1651. DOI: 10.1016/S0002-9440(10)65035-3.
13. Kryklyva V, ter Liden E, Kroeze L, de Voer RM, van der Kolk BM, Stommel MWJ, Hermans JJ, Luchini C, Wood LD, Hruban RH, Nagtegaal ID, Ligtenberg MJL, Brosens LAA. Medullary Pancreatic Carcinoma Due to Somatic POLE Mutation. A Distinctive Pancreatic Carcinoma With Marked Long-Term Survival. Pancreas 2020; 49(7): 999–1003. DOI: 10.1097/MPA.0000000000001588.
14. Verocq C, Racu ML, Bafort D, Butorano G, Perez-Casanova Garsia L, Navez J, Witterwulghe M, Sheahan K, Swan N, Closset J, van Laethem JL, Maris C, D’Haene N. Pancreatic medullary carcinoma developed on a pancreatic intraductal papillary mucinous neoplasm with loss of MSH2 and MSH6 expression: a case report. Diagn Pathol 2021; 13(16): 117. DOI: 10.1186/s13000-021-01178-0.
15. Holloman C, Carlan SJ, Sundharkrishnan L, Guzman A, Madruga M. Successful pregnancy after mucinous cystic neoplasm with invasive carcinoma of the pancreas in a patient with polycystic ovarian syndrome: a case report. J Med Case Rep 2017; 11(11): 188. DOI: 10.1186/s13256-017-1343-y.
16. Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY, Mino-Kenudson M, Capelli P, Pederzoli P, Fernández-del Castillo. Mucinous Cystic Neoplasm of the Pancreas is Not an Aggressive Entity. Ann Surg 2008; 247(4): 571–579. DOI: 10.1097/SLA.0b013e31811f4449.
17. Iusco DR, Navarra G, Bonomi S, Grassi A, Vicari S, Virzì S. Pancreatic large mucinous cystoadenoma with invasive ductal carcinoma in pregnancy. Case report. G Chir 2012; 33(5): 163–167.
18. Nishi T, Kawabata Y, Ishikawa N, Araki A, Yano S, Maruyama R, Tajima Y. Intraductal papillary mucinous carcinoma of the pancreas associated with pancreas divisum: a case report and review of the literature. BMC Gastroenterol 2015; 8(15): 78. DOI: 10.1186/s12876-015-0313-3.
19. Shindo K, Ueda J, Aishima S, Aso A, Ohtsuka T, Takahata S, Ishigami K, Oda Y, Tanaka M. Small-Sized, Flat-Type Invasive Branch Duct Intraductal Papillary Mucinous Neoplasm: A Case Report. Case Rep Gastroenterol 2013; 7(3): 449–454. DOI: 10.1159/000355939.
20. Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM, Akisik FM, Sherman S, Pitt HA, Lillemoe KD. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg 2007; 246(4): 644–654. DOI: 10.1097/SLA.0b013e318155a9e5.
21. Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K, International Association of Pancreatology. International Association of Pancreatology International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12(3): 183–197. DOI: 10.1016/j.pan.2012.04.004.
22. Yamao K, Yanagisawa A, Takahashi K, Kimura W, Doi R, Fukushima N, Ohike N, Shimizu M, Hatori T, Nobukawa B, Hifumi M, Kobayashi Y, Tobita K, Tanno S, Sugiyama M, Miyasaka Y, Nakagohri T, Yamaguchi T, Hanada K, Abe H, Tada M, Fujita N, Tanaka M. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas 2011; 40(1): 67–71. DOI: 10.1097/MPA.0b013e3181f749d3.
23. WHO Classification of Tumours Editorial Board. Digestive System Tumours (5th edition). IARC. Vol. 1. Lyon, 2019.
24. Hui L, Rashid A, Foo WC, Katz MH, Chatterjee D, Wang H, Fleming JB, Tamm EP, Wang H. Significance of T1a and T1b Carcinoma Arising in Mucinous Cystic Neoplasm of Pancreas. Am J Surg Pathol 2018; 42(5): 578–586. DOI: 10.1097/PAS.0000000000001040.
25. Makino H, Kametaka H, Fukada T, Seike K, Hasegawa A, Isozaki M. Long-Term Survival in Response to Multimodality Therapy in a Patient with Invasive Pancreatic Cancer with Cyst Formation. Gan To Kagaku Ryoho 2016; 43(12): 1997–1999.
26. Lewis GH, Wang H, Bellizzi AM, Klein AP, Askin FB, Schwartz LE, Schulick RD, Wolfgang CL, Cameron JL, O’Reilly EM, Yu KH, Hruban RH. Prognosis of Minimally Invasive Carcinoma Arising in Mucinous Cystic Neoplasms of the Pancreas. Am J Surg Pathol 2013; 37(4): 601–605. DOI: 10.1097/PAS.0b013e318273f3b0.
