Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2023, vol. 22(1), pp. 33–40 DOI: https://doi.org/10.15388/LietChirur.2023.22.68
Reliability of the Lymph Node Ratio in the Prediction of Gastric Cancer Survival
Sedat Kamalı
Department of General Surgery, Prof. Dr. Cemil Taşçıoğlu Şehir Hastanesi, Istanbul, Turkey
E-mail: kamalisedat@gmail.com
Cemal Ulusoy
Department of General Surgery, Prof. Dr. Cemil Taşçıoğlu Şehir Hastanesi, Istanbul, Turkey
E-mail: drculusoy@gmail.com
Gülçin Harman Kamalı
Department of Pathology, Prof. Dr. Cemil Taşçıoğlu Şehir Hastanesi, Istanbul, Turkey
E-mail: kamaligulcin@yahoo.com.tr
Abstract. Background. Lymph node metastasis is the major determinant factor in the prognosis of gastric cancer. There is still no definite consensus on the lymph node number that should be harvested during gastric cancer surgery. Lymph Node Ratio (LNR) is defined as the ratio of metastatic nodes to the total number of pathologically examined lymph nodes. LNR has been proposed to be a sensitive prognostic factor in patients with gastric cancer. In this study the reliability of the LNR is tested for being a prognostic factor in gastric cancer survival. Methods. Medical records of 244 patients, with neither distant metastases nor neoadjuvant treatment underwent curative gastrectomy, were analyzed retrospectively in terms of survival according to the lymph node ratio (LNR). Patients were divided in two groups by using LNR cut-off value. Results. LNR of 0.4 was proved to be the best cut-off value to predict the prognosis of patients with gastric cancer. Univariate and multivariate analysis revealed that age over 65 (p < 0.001), and LNR ≥ 0.4 (p = 0.02) were independent factors in gastric cancer survival. Patients with LNR ≥ 0.4 presented with worse outcomes regarding other prognostic parameters (tumor differentiation, tumor diameter, lymphovascular invasion or perineural invasion), despite similar numbers of lymph nodes being harvested in both groups during surgery. Conclusion. Lymph node ratio is a reliable parameter to predict the survival in gastric cancer.
Key words: gastric cancer, lymph node ratio, prognosis.
Received: 2023/02/18. Accepted: 2023/03/01.
Copyright © 2023 Sedat Kamalı, Cemal Ulusoy, Gülçin Harman Kamalı. Published by Vilnius University Pr ess. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Gastric cancer remains the fifth most common and fourth deadliest cancer type [1]. Despite the latest surgical and/or medical treatments, long-term survival has not been improved satisfactorily [2]. Gastric cancer is a locoregional disease with high lymphatic metastasis rates [3]. Surgery has been proved as the sole curative approach. However, high locoregional relapse and distant metastases are still one of the many prognosis-determining factors and currently there is no established therapy for eventual disease recurrence. Despite all parameters, tumor staging is the crucial value such as tumor invasion (T stage) and lymph node invasion (N stage). The presence of lymphatic metastases is one the most important prognostic factors even if there is no standardized concept [4].
Various studies such as Bando Zu et al., Kano et al. and Hou et al. showed the ratio of metastatic lymph nodes to total harvested lymph nodes (LNR) to be an independent prognostic factor [4–9]. This study is aimed to show the prognostic value of lymph node ratio in the prognosis of the non-metastatic gastric cancer patients in any curative resectable stage after defining the cut-off ratio of 0.4 as suggested by Komatsu et al. [7]. This study compared these patients in terms of their survival rate.
Methods
This retrospective cohort study was approved by the ethics committee of Prof. Dr. Cemil Tascioglu Sehir Hastanesi (Protocol No. E-48670771-514.99, decision number 86).
Non-metastatic gastric cancer patients who underwent surgery without neoadjuvant therapy conduction were recruited between 2006 and 2013. Patients’ medical data and pathology reports were retrospectively analyzed. Demographic data (age, gender), tumor characteristics (location, diameter, histopathological differentiation, lymphovascular invasion (LVI), perineural invasion (PNI)), total number of harvested lymph nodes and number of positive lymph nodes were recorded.
Patients without preoperative metastasis and/or synchronous tumor were included in the study. Exclusion criteria comprehended patients with harvested less than 15 lymph nodes, positive resection margins and mortality within the first 30 days after surgery. LNR, age and tumor size cut-off value were specified by using the receiver operating characteristic (ROC) analysis.
Patients were divided in two groups by using an LNR cut-off value of 0.4. LNR0 group for the value of LNR < 0.4 and LNR1 group for the value of LNR ≥ 0.40, respectively.
Patients who required adjuvant therapy were referred to the oncology department for treatment within the first six weeks of resection. For 6 to 8 cycles, patients were treated with a 5-fluorouracil-based (FU–FA) regimen or capecitabine plus oxaliplatin (XELOX). SPSS 16.0.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Clinical and pathological parameters were analyzed with Chi-square test. Mann Whitney-U test, Kaplan Meier and Multivariable Cox Proportional Hazards, ROC tests. Statistical significance was set for p value of ≤0.05.
Results
Of 81 female (33%) and 163 male (67%) patients with a median age of 65 (29–93), the tumor localization was: cardia, fundus in 59 (24.2%), body of stomach in 79 (32.4%), antrum in 94 (38.5%) and linitis plastica in 12 (4.9%) patients.
The median tumor size was 6 cm (1.5–23 cm). According to the TNM 8th ed. staging, 35 patients were T1 (14.3%), 61 patients T2 (25%), 133 patients T3 (54.5%) and 15 patients T4 (6.1%) respectively. 54 patients had N0 status (22.1%), 44 patients had N1 (18%), 39 patients had N2 (16%) and 107 patients N3 (43.9%), respectively. Median harvested lymph node number was 24 (15–74) and median positive lymph node number was 5 (0–74).
Histopathological grade was 13 (5.3%) patients with well-differentiated tumor, 102 (41.8%) patients with moderately differentiated, 104 (42.6%) patients with poorly differentiated, 5 (2%) patients presented with mucinous carcinoma and 20 (8.2%) patients with signet ring cells. There were 169 (69.3%) patients with LVI and 136 (55.7) patients with PNI.
LNR was defined as the ratio of metastatic lymph nodes to the total number of harvested lymph nodes. In order to find the most adequate cut off value, Area Under Curve (AUC) value was found as 0.65 for overall survival rate. For the optimal LNR cut off value was 0.4, sensitivity and specificity were 80% and 47%, respectively. Patients were divided in two groups by using an LNR cut-off value of 0.4. LNR0 group for the value of LNR < 0.4 and LNR1 group for the value of LNR ≥ 0.40, respectively.
Similar cut-off values were also analyzed for the age of 65 (AUC: 0.69/64.5 years/56% sensitivity/69% specificity) and 6 cm for the tumor size (AUC: 0.61/5.8cm/65% sensitivity/51% specificity) (Figure 1).
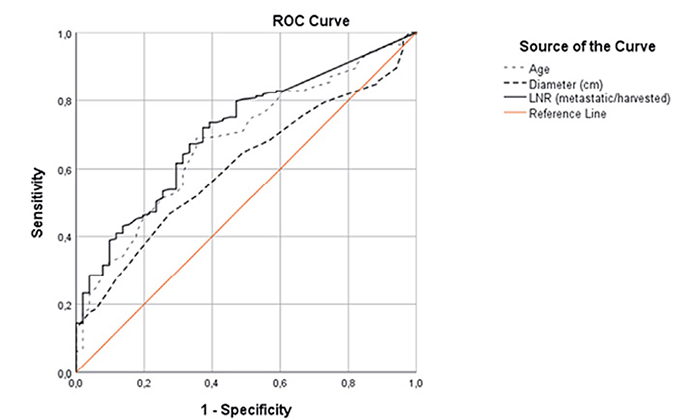
Figure 1. ROC Curve for the optimal cut-off value (for LNR, tumor diameter and age)
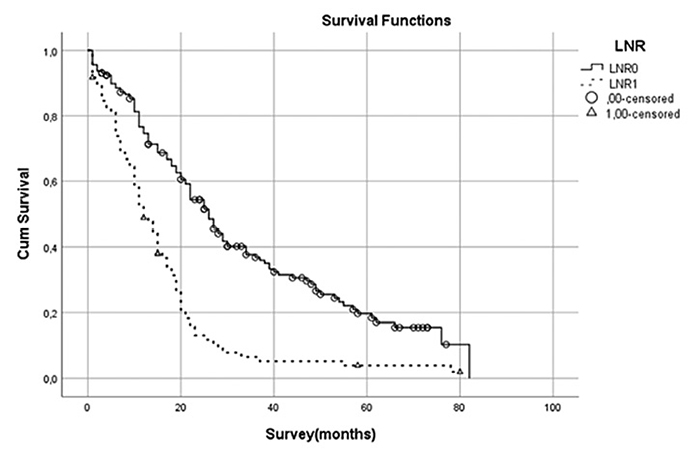
Figure 2. Survival analysis for LNR cut-off value of 0.4 presented with worse outcome for the LNR1 group (p < 0.001)
Mean follow up time was 55.5 months (1–195; SD: 58.4). Mean survival time was 150.9 months for the postoperatively deceased patients. Mean follow-up period was 30.3 months for the surviving patients.
There was no statistically significant difference in survival between the genders. However, when the subgroups were divided according to the median age of 65, there was a shorter survival time in patients over 65 (p = 0.04). The mean survival period for patients diagnosed with linitis plastica showed shorter survival (26.2 months) but without statistical difference (p = 0.13). Patients with a T1 stage showed mean survival of 86.1 (median: 84.7; SD: 65.3; 1–195) months, whereas T4 stage patients had mean 28.4 (median: 11.4; SD: 42; 1–138) months survival period, which was statistically significant (p < 0.001). According to N stage, N0 patients survived up to mean 87.2 months and N3 patients up to mean 31.6 months (p < 0.001). Survival time also differed between the patients with high grade tumors (mean 125 months) and the patients with signet ring cell tumor (mean 42.5 months) (p = 0.02).
The LNR0 and LNR1 group also had statistically significant differences in survival time (p < 0.001) (Figure 2).
Among all the factors influencing the survival time, according to the multivariate analysis (Cox regression), in patients with the age over 65, LNR over 0.4 showed the highest impact on survival (Table 1).
Table 1. Prognostic factors in gastric cancer
|
|
n |
Univariateb |
Multivariatec |
|||
|
HR |
(95 % CI) |
p value |
||||
|
Age |
≥65 years/<65 years |
124/120 |
0.04 |
1.9 |
1.4–2.6 |
<0.001a |
|
Gender |
Male/Female |
163/81 |
0.52 |
|
– |
|
|
Localization |
Proximal/Corpus/ |
59/79/94/12 |
0.13 |
|
– |
|
|
Histological type |
Well/moderately diff./ |
13/102/104/5/20 |
0.02 |
|
|
0.38 |
|
Tumor |
>60 mm/<60 mm |
150/94 |
0.04 |
1.2 |
0.91–1.68 |
0.19 |
|
pT |
T1/T2/T3/T4 |
35/61/133/15 |
<0.001 |
|
|
0.15 |
|
pN |
N0/N1/N2/N3 |
54/44/39/107 |
<0.0001 |
|
|
0.42 |
|
Lymphovascular invasion |
LVI (+)/LVI (–) |
168/76 |
<0.001 |
1.1 |
0.67–1.9 |
0.64 |
|
Perineural invasion |
PNI (+)/PNI (–) |
136/108 |
0.06 |
1.1 |
0.77–1.5 |
0.67 |
|
LNRa |
LNR1/LNR0 |
85/159 |
<0.001 |
1.8 |
1.1–2.8 |
0.02a |
a – statistically significant factors in both univariate and multivariate analysis; b – univariate analysis; c – application of the Cox proportional hazards model.
The LNR1 patients presented mostly with tumors such as linitis plastica (p < 0.001), with more undifferentiated tumor cells (signet ring cell), higher tumor diameter and more tumors in the T4 stages than T1, T2 and T3. At the same time, in the LNR1 group, more metastatic lymph nodes with present LVI and PNI (p < 0.001) were noted. In both LNR0 and LNR1 groups, similar numbers of lymph nodes were resected (Table 2).
Table 2. Characteristics after LNR 0.4 subgrouping
|
|
LNR1 |
LNR0 |
p value |
|
|
Age |
Median (range) |
62 (38–88) |
67 (36–93) |
0.09a |
|
Gender |
Male/Female |
58/27 |
105/54 |
0.421b |
|
Tumor site |
Proximal/Corpus/Antrum/ |
14/27/34/10 |
45/52/60/2 |
0.001b |
|
Histological type |
Well/moderately diff./ |
0/27/45/0/13 |
13/75/59/5/7 |
<0.001b |
|
Tumor diameter (mm) |
Median (range) |
70 (2–88) |
60 (15–160) |
0.001a |
|
T-stage |
T1/T2/T3/T4 |
8/13/55/9 |
27/48/78/6 |
<0.001b |
|
Lymphovascular invasion (LVI) |
–/+ |
6/79 |
70/89 |
<0.001b |
|
Perineural invasion (PNI) |
–/+ |
20/65 |
88/71 |
<0.001b |
|
Number of metastatic |
Median (range) |
17 (6–74) |
2 (0–11) |
<0.001a |
|
Total number of lymph |
Median (range) |
24 (15–74) |
23 (15–60) |
0.39a |
a – Mann–Whitney U test; b – Chi-square test.
Discussion
Regardless of the tumor location, it is crucial to extract as many lymph nodes as possible for a R0 resection. The depth of the tumor invasion shows a tendency to correlate with the number of lymph node metastases. Generally, the tumor invasion depth and the number of metastatic lymph nodes are accepted as the most important prognostic factors [10–15]. Union of International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC) also base their staging on the number of metastatic lymph nodes [16].
In gastric cancer, determining prognostic factors play an important role in choosing the most appropriate treatment modality. The number of extracted lymph nodes can vary, even being extracted via the same technique every time and can be influenced by many factors. There are different lymphadenectomy methods described and preferred in Eastern and Western countries as a consequence of epidemiological differences, such as obesity rates and other risk factors for gastric cancer [17–21]. The surgeons’ experience, surgical technique (e.g., D1 vs. D2 dissection) and biological factors determine the number of resected lymph nodes. Higher body-mass-index (BMI) is associated with fewer lymph nodes. Adipose tissue, anatomical variations, inexperienced surgeon and unthorough histopathological tissue processing also led to lower number of extracted lymph nodes [22]. Low number of extracted lymph nodes might eventually lead to inaccurate staging of the disease and under staging [15, 23]. Up to 5–15% of the cases, a stage migration in the TNM classification according to the AJCC might be seen, due to this bias [24].
Correct application of the LNR can reduce this bias and the international differences in the diagnostics, staging and treatment. An international standardized approach would also improve the prognosis of gastric cancer. However, various cut-off values (0.4–0.75) are reported in the literature [24]. Some groups adopted the 0.4 ratio, which was also defined in this study [7]. In various studies some authors are suggesting leveled cut-off value instead of one level cut-off value and some authors preferred to find cut-off value in stage III cancer group. In this study stage is not researched and the operations performed by the different surgeons because of this one level cutoff is found in the researched study group [25]. In this study the feasibility of the LNR as a prognostic factor in all kinds of surgeries regardless of their lymph node stages was proved.
While patients’ age (≥65 years/<65 years), histologic differentiation (well, moderately, poorly differentiated, signet ring cell tumors), tumor size (≥60 mm/<60 mm), T stage (T1/T2/T3/T4), LVI (+/–), PNI (+/–), LNR (≥0.4/<0.4) showed statistical significance in the univariate analysis. On the other hand, age and LNR ≥ 0.4 affected survival time statistically significant in the multivariate analysis (Table 1).
In their univariate analysis Komatsu et al. [7] found that the age, tumor size, T and N stages, LVI, PNI and LNR affected the survival period, whereas only age, tumor size and LNR of the N3 stage of the gastric cancers appeared to be the independent factors in multivariate analysis. The multivariate analysis in this study showed that patients’ age and LNR ≥ 0.4 are independent prognostic factors regardless of subgrouping of the lymph nodes.
LNR becomes more important, in patients with insufficient lymph node dissection, for prediction of prognosis.
Recent articles proved an increase of incidence in young onset gastric cancer cases and prognosis is worse than the late onset patients [26]. However, in this study LNR high groups are more common in young patients.
In patients with gastric cancer, LNR represents tumor features (number of metastatic lymph nodes) and also acts as a treatment parameter (extent of lymph node dissection) [27]. Although the number of extracted lymph nodes in both of the subgroups divided according to the LNR ≥ 0.4 is similar, this is specified in similar articles [25].
Linitis plastica affects survival in univariate analysis but in multivariate analysis there is no effect on survival. However, linitis plastica is more common in the LNR1 group. In the event of a rise in the number of cases, linitis plastica may be one of the variables affecting survival independently.
Other factors such as LVI (+), PNI (+), and T4 tumors have been found in studies to have a worse prognosis, but a statistically significant greater incidence in LNR (+) groups has been documented, with deterioration in patients with high LNR (+) described as prognosis [28–30].
One explanation for this correlation is because LNR is apparently linked to the possibility of the patient’s immune reactivity as a defensive mechanism to be a more complex prognostic marker that takes into account both, surgical efficacy and cancer biology and the spread of malignant cells [18–22]. For example, the status of epidermal growth factor receptor (EGFR) expression, which controls the immune response by influencing the activity of regulatory T-cells or myeloid-derived suppressor cells during cancer development, is apparently linked to immunological reactivity [9, 31, 32]. Additionally, Oh et al. discovered that EGFR mutations were linked to advanced illness [33].
Lymph node number and pN stage can be related with LNR but in gastric cancer there are still cellular mechanisms which might produce different results in future studies and yet need to be proven.
Limitations
This study is retrospective and the number of patients is relatively small. The standards in surgical techniques are not uniform and show varieties. Many different authors stated different cut-off values for LNR other than the one in this study. Neoadjuvant therapy was not applied, although it has been used in recent years.
Conclusion
Patients’ age, T stage and N stage are statistically significant factors that influence survival. The presence of LVI and PNI in tumors also affected the prognosis. LNR ≥ 0.4 is associated with worse prognosis. It can contribute to the decisions on the postoperative therapy regimes of patients, where an extended lymph node dissection cannot be performed for different reasons. To conclude, LNR is a reliable determinant of the prognosis in gastric cancer.
Conflict of interest statement. The authors of this work have nothing to disclose.
Ethics statement. The study was approved by the local ethics committee of the clinic, Protocol No. E-48670771-514.99, decision number 86.
Funding statement. Funding information is not available.
Acknowledgement. We would like to express gratitude to Assoc. Prof. Dr. Sedat Kamalı, the co-author of this article. He is passed away after completing and before submitting this article. Dr. Kamalı dedicated his life surgery, education of surgery and science. May he rest in peace.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209–249.
2. Hoshi H. Management of Gastric Adenocarcinoma for General Surgeons. Surg Clin North Am 2020; 100(3): 523–534.
3. Tan Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit 2019; 13(25): 3537–3541.
4. Hou Y, Wang X, Chen J. Prognostic significance of metastatic lymph node ratio: the lymph node ratio could be a prognostic indicator for patients with gastric cancer. World J Surg Oncol 2018; 16(1): 198.
5. Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 2002; 9: 775–784.
6. Xu DZ, Geng QR, Long ZJ, Zhan YQ, Li W, Zhou ZW, Chen YB, Sun XW, Chen G, Liu Q. Positive lymph node ratio is an independent prognostic factor in gastric cancer after d2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol 2009; 16(2): 319–326.
7. Komatsu S, Ichikawa D, Miyamae M, Kosuga T, Okamoto K, Arita T, Konishi H, Morimura R, Murayama Y, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Fujiwara H, Otsuji E. Positive Lymph Node Ratio as an Indicator of Prognosis and Local Tumor Clearance in N3 Gastric Cancer. J Gastrointest Surg 2016; 20(9): 1565–1571.
8. Kılıç MÖ, Gündoğdu SB, Özden S, Saylam B, Tez M. The prognostic value of different node staging systems in patients with ≤15 lymph nodes following surgery for gastric adenocarcinoma. Acta Chir Belg 2018; 118(1): 1–6.
9. Kano K, Yamada T, Yamamoto K, Komori K, Watanabe H, Hara K, Shimoda Y, Maezawa Y, Fujikawa H, Aoyama T, Tamagawa H, Yamamoto N, Cho H, Shiozawa M, Yukawa N, Yoshikawa T, Morinaga S, Rino Y, Masuda M, Ogata T, Oshima T. Association Between Lymph Node Ratio and Survival in Patients with Pathological Stage II/III Gastric Cancer. Ann Surg Oncol 2020; 27(11): 4235–4247.
10. Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer 1998; 1: 125–133.
11. Yamamura Y, Nakajima T, Ohta K, Nashimoto A, Arai K, Hiratsuka M, Sasako M, Kodera Y, Goto M. Determining prognostic factors for gastric cancer using the regression tree method. Gastric Cancer 2002; 5(4): 201–207.
12. Fukuda N, Sugiyama Y, Midorikawa A, Mushiake H. Prognostic significance of the metastatic lymph node ratio in gastric cancer patients. World J Surg 2009; 33(11): 2378–2382.
13. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021; 71(3): 264–279.
14. Yaprak G, Tataroglu D, Dogan B, Pekyurek M. Prognostic factors for survival in patients with gastric cancer: Single-centre experience. North Clin Istanb 2019; 7(2): 146–152.
15. Che K, Wang Y, Wu N, Liu Q, Yang J, Liu B, Wei J. Prognostic Nomograms Based on Three Lymph Node Classification Systems for Resected Gastric Adenocarcinoma: A Large Population-Based Cohort Study and External Validation. Ann Surg Oncol 2021; 28(13): 8937–8949.
16. O’Sullivan B, Brierley J, Byrd D, Bosman F, Kehoe S, Kossary C, Piñeros M, van Eycken E, Weir HK, Gospodarowicz M. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol 2017; 18(7): 849–851.
17. Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340(12): 908–914.
18. Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, Coit DG, Brennan MF. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010; 251(4): 640–646.
19. Bickenbach K, Strong VE. Comparisons of Gastric Cancer Treatments: East vs. West. J Gastric Cancer 2012; 12(2): 55–62.
20. Yamamoto M, Rashid OM, Wong J. Surgical management of gastric cancer: the East vs. West perspective. J Gastrointest Oncol 2015; 6(1): 79–88.
21. Russo A, Li P, Strong VE. Differences in the multimodal treatment of gastric cancer: East vs. West. J Surg Oncol 2017; 115(5): 603–614.
22. Asoglu O, Matlim T, Kurt A, Onder SY, Kunduz E, Karanlik H, Sam B, Kapran Y, Bugra D. Guidelines for extended lymphadenectomy in gastric cancer: a prospective comparative study. Ann Surg Oncol 2013; 20(1): 218–225.
23. Marchet A, Mocellin S, Ambrosi A, de Manzoni G, Di Leo A, Marrelli D, Roviello F, Morgagni P, Saragoni L, Natalini G, De Santis F, Baiocchi L, Coniglio A, Nitti D, on behalf of the Italian Research Group for Gastric Cancer Study (GIRCG). The prognostic value of N-ratio in patients with gastric cancer: validation in a large, multicenter series. Eur J Surg Oncol 2008; 34(2): 159–165.
24. Nakamura S, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Tanaka C, Kobayashi D, Fujiwara M, Murotani K, Kodera Y. Accurate Risk Stratification of Patients with Node-Positive Gastric Cancer by Lymph Node Ratio. World J Surg 2020; 44(12): 4184–4192.
25. Gulmez S, Senger AS, Uzun O, Omeroglu S, Sert ZO, Oz A, Polat E, Duman M. Prognostic significance of the metastatic lymph node ratio compared to the TNM classification in stage III gastric cancer. Niger J Clin Pract 2021; 24(11): 1602–1608.
26. Jiang Y, Huang W, Xie J, Han Z, Chen C, Xi S, Sun Z, Hu Y, Zhao L, Yu J, Li T, Zhou Z, Cai S, Li G. Young age increases risk for lymph node positivity in gastric cancer: A Chinese multi-institutional database and US SEER database study. J Cancer 2020; 11(3): 678–685.
27. Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J Clin Oncol 2005; 23(28): 7114–7124.
28. Erstad DJ, Blum M, Estrella JS, Das P, Minsky BD, Ajani JA, Mansfield PF, Ikoma N, Badgwell BD. Benchmarks for nodal yield and ratio for node-positive gastric cancer. Surgery 2021; 170(4): 1231–1239.
29. Wang PL, Huang JY, Zhu Z, Gong BC, Huang HW, Duan SJ, Xu HM, Liu FN. Development of a risk-scoring system to evaluate the serosal invasion for macroscopic serosal invasion positive gastric cancer patients. Eur J Surg Oncol 2018; 44(5): 600–606.
30. Lee HH, Song KY, Park CH, Jeon HM. Undifferentiated-type gastric adenocarcinoma: prognostic impact of three histological types. World J Surg Oncol 2012; 10: 254.
31. Zaiss DM, van Loosdregt J, Gorlani A, Bekker CPJ, Gröne A, Sibilia M, van Bergen en Henegouwen PMP, Roovers RC, Coffer PJ, Sijts AJAM. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013; 38(2): 275–284.
32. Thakur A, Schalk D, Tomaszewski E, Kondadasula SV, Yano H, Sarkar FH, Lum LG. Microenvironment generated during EGFR targeted killing of pancreatic tumor cells by ATC inhibits myeloid-derived suppressor cells through COX2 and PGE2 dependent pathway. J Transl Med 2013; 11: 35.
33. Oh HS, Eom DW, Kang GH, Ahn YC, Lee SJ, Kim JH, Jang HJ, Kim EJ, Oh KH, Ahn HJ. Prognostic implications of EGFR and HER-2 alteration assessed by immunohistochemistry and silver in situ hybridization in gastric cancer patients following curative resection. Gastric Cancer 2014; 17(3): 402–411.