Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2023, vol. 22(3), pp. 167–172 DOI: https://doi.org/10.15388/LietChirur.2023.22(3).6
Heterotopic Ossification in the Abdomen of an Elderly Patient, as an Incidental Finding During Elective Surgery: A Case Report
Gordana Bozhinovska-Beaka
City General Hospital “8th of September”, Skopje, Republic of North Macedonia
Faculty of Medicine, Goce Delcev University, Shtip, Republic of North Macedonia
E-mail: g.bozinovska.beaka@gmail.com
Biljana Prgova Veljanova
City General Hospital “8th of September”, Skopje, Republic of North Macedonia
Faculty of Medicine, Ss. Cyril and Methodius University in Skopje, Skopje, Republic of North Macedonia
E-mail: biljana.veljanova@gmail.com
Tatjan Troik
City General Hospital “8th of September”, Skopje, Republic of North Macedonia
Faculty of Medicine, Goce Delcev University, Shtip, Republic of North Macedonia
E-mail: tatjanatroik@gmail.com
Ivica Stojanovski
City General Hospital “8th of September”, Skopje, Republic of North Macedonia
Faculty of Medicine, Goce Delcev University, Shtip, Republic of North Macedonia
E-mail: ivica.stojanovski@gmail.com
Patricija Kalamaras
Clinical Hospital “Acibadem Sistina”, Skopje, Republic of North Macedonia
Faculty of Medicine, Goce Delcev University, Shtip, Republic of North Macedonia
E-mail: patricijakalamaras@gmail.com
Nadica Bozhinovska-Dimova
Clinical Hospital “Acibadem Sistina”, Skopje, Republic of North Macedonia
E-mail: nadica.dimova@gmail.com
Abstract. Introduction. Heterotopic ossification (HO) is a rare diverse pathologic process, with broad spectrum. Case report. This is a case report of a 73 years old patient who had ventral hernia at the medial line on the anterior abdominal wall, and underwent a routine surgery. During the surgery an incidental finding of HO was discovered. Discussion. HO is well documented to occur at increased frequency with certain predisposing conditions, including orthopedic surgery, most commonly hip arthroplasty. Non-genetic HO can occur nearly anywhere in the body, but is often designated by the tissue type it involves, such as myositis ossificans when involving skeletal muscle, or fasciitis ossificans when involving fascia.
Key words: heterotopic ossification, abdominal surgery, ventral hernia, osteosarcoma.
Received: 2023/07/19. Accepted: 2023/08/30.
Copyright © 2023 Gordana Bozhinovska-Beaka, Biljana Prgova Veljanova, Tatjan Troik, Ivica Stojanovski, Patricija Kalamaras, Nadica Bozhinovska-Dimova. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Heterotopic ossification (HO) is a diverse pathologic process, defined as the formation of extra skeletal bone in muscle and soft tissues. The word “heterotopic” is derived from the Greek roots “hetero” and “topos”, meaning “other place”. HO can be conceptualized as aberrant tissue repair and is increasingly recognized as a common complication of trauma, surgery, and other local or systemic insults. Non-genetic forms of HO are most common, but rare genetic forms of HO also exist. The spectrum of HO is broad. Some HO lesions may be small and clinically irrelevant, while others may exact a high morbidity [1]. Here we present a case report of HO, as an incidental finding during abdominal surgery.
Case report
A 73 years old patient has been hospitalized for elective ventral hernia surgery. His medical history shows that 2 years prior, he was surgically treated for malignancy of the cecum, and left hemi-colectomy was performed. Since then, he has done all the treatments needed. Furthermore, he has high blood pressure and type 2 diabetes mellitus, for which he takes regular antihypertensive and antidiabetic therapy (Enalapril 5 mg, and Metformin 1000 mg). The current hospitalization is because a surgery is needed in order to treat ventral hernia that occurred at the site of the surgical scar (medial line) on the anterior abdominal wall. On admission, the patient’s abdomen was at the same level as his chest, soft and painless on palpation, and a ventral hernia the size of a citron has been noted, situated on the medial scar from the previous operation (from 2 years ago), the hernia was easily repositionable. His laboratory findings and his chest X-ray showed no abnormal finding. During the surgery, at the very opening of the abdominal cavity along the line of the old surgical scar, a hard structure was found along the very edge of the incision, located intramuscularly. The structure, which was 13 cm long, was prepared and safely removed from the abdominal wall, and sent for pathological examinations, per protocol (Figures 1–3). The ventral hernia was surgically treated as well, and the patient was transported to the post-operative intensive care unit. The pathohistological finding corresponded to heterotopic ossification in the abdominal wall. The period after the surgery went without complications, due to the age, the patient spent total of 9 days in the hospital, and on the 8th post-operative day he was discharged. Full body X-ray was performed after surgery, no ill-placed bone tissue was found.
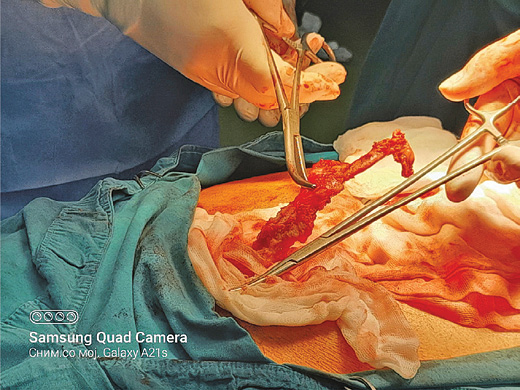
Figure 1. During the surgery, at the very opening of the abdominal cavity a hard structure 13 cm long was found along the very edge of the incision, located intramuscularly
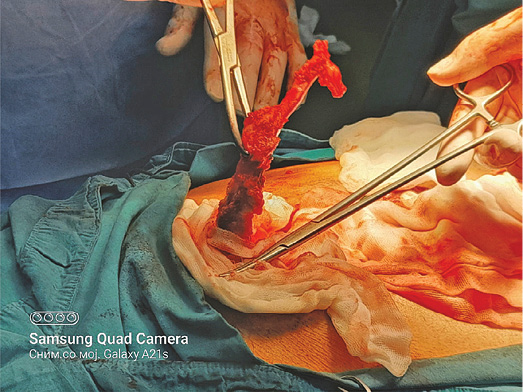
Figure 2. During the surgery, at the very opening of the abdominal cavity a hard structure 13 cm long was found along the very edge of the incision, located intramuscularly
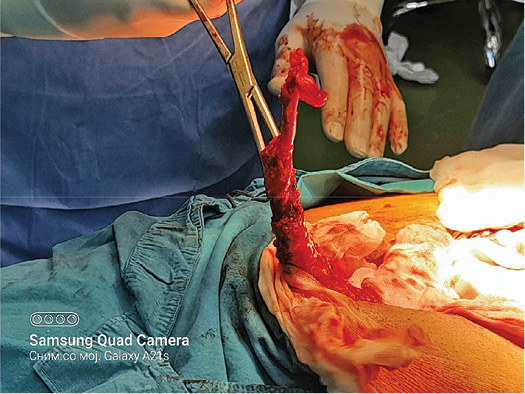
Figure 3. During the surgery, at the very opening of the abdominal cavity a hard structure 13 cm long was found along the very edge of the incision, located intramuscularly
Pathohistology
Microscopically, mature cortical and trabecular bone can be seen on the samples taken. The bone trabeculae are thin and widely separated, and between them is bone marrow, built of mature adipose tissue with multifocal hematopoietic activity. Peripherally, the cortical bone is adjoined by collagenous connective tissue and mature adipose tissue, with and easily expressed lymphocytic inflammatory infiltrate with perivascular distribution. In one part of the surrounding connective tissue, the process of forming new bone tissue, represented by primary bone tissue, can be seen (Figures 4–7).
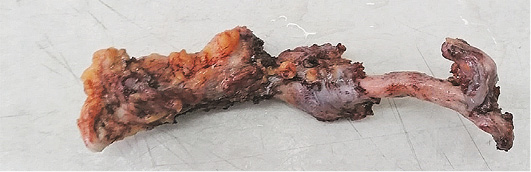

Figure 4. Fragment of bone removed from the area of previous surgery in the mid-abdominal wall. The specimen grossly consisted of bone measuring 13 cm in the greatest dimension
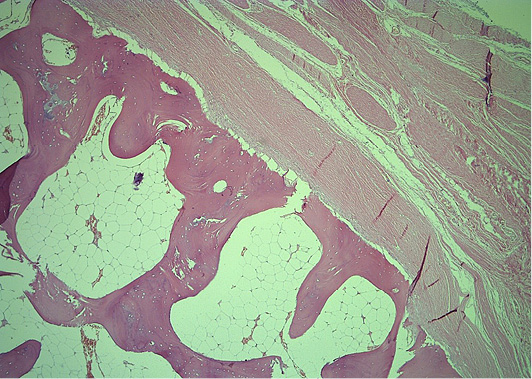
Figure 5. Histopathology revealed mature bone tissue with bone marrow and peripheral fibrous tissue, with no evidence of atypia or malignancy (He&Eo staining. Magnification ×40)
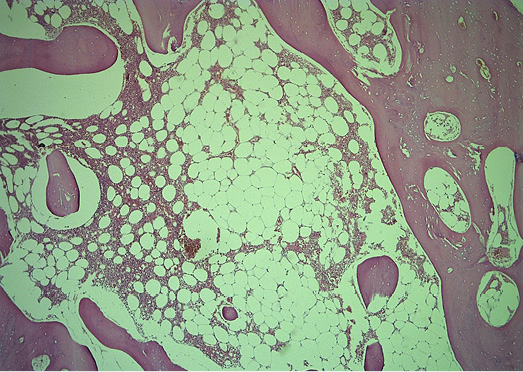
Figure 6. Histologic section of the HO showing mature bone tissue and bone marrow with fat cells and haematopoiesis (He&Eo staining. Magnification ×40)
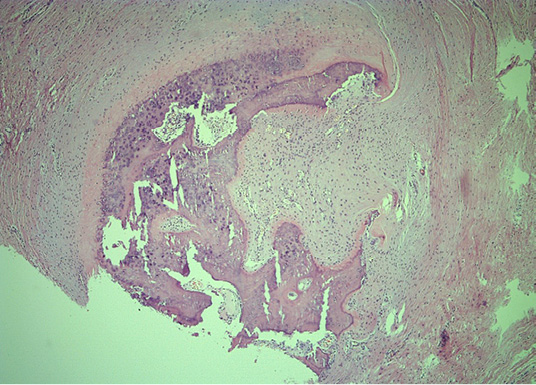
Figure 7. Representative peripheral area, demonstrating woven bone and compressed fibrous pseudocapsule on the lesions’ exterior (He&Eo staining. Magnification ×40)
Discussion
HO is well documented to occur at increased frequency with certain predisposing conditions, including orthopedic surgery, most commonly hip arthroplasty (occurs in up to ~40% of cases) [2–4]. Approximately half of patients are in their second and third decades of life; however, a broad age distribution is present from infancy to late adulthood [5]. Men are slightly more commonly affected with a sex ratio of 3:2. Non-genetic HO can occur nearly anywhere in the body, but the most common areas include locations that are susceptible to trauma, such as the elbow, thigh, pelvis, and shoulder [6, 7]. Non-genetic HO is often designated by the tissue type it involves, such as myositis ossificans when involving skeletal muscle, or fasciitis ossificans when involving fascia. Myositis ossificans is the most common term used among pathologists, although this term is a misnomer when more broadly used to discuss HO (as HO is neither specific to muscle nor involves prominent inflammation after its early stages) [8].
Early lesions are often hyper cellular and with little bone matrix and can prompt concern for soft‐tissue sarcoma. Later lesions have prominent bone formation with a characteristic zonal architecture. This zonal architecture with a predominant peripheral ossification is a hallmark of HO. Early lesions demonstrate a hypercellular proliferation of spindle cells often with little bone matrix. Spindled areas often have features of “nodular fasciitis” or “granulation tissue”, including high numbers of normal mitotic figures, scattered multinucleated giant cells, scattered inflammatory cells, and extravagated red blood cells. As ossification ensues, woven bone with prominent osteoblastic cell lining is characteristic. A gradual continuum of woven bone to more mature lamellar bone is often found, one helpful feature to distinguish HO from extra skeletal osteosarcoma. HO is well circumscribed, with a surrounding fibrous pseudo capsule often with thick‐walled blood vessels [9].
More mature lesions resemble native bone elements, including thickened trabeculae of lamellar bone with central fatty marrow and vascular spaces resembling bone marrow sinusoids. The peripheral aspects of mature HO may demonstrate compact bone tissue, which resembles native cortical bone, sometimes including histologic features reminiscent of Haversian systems and Volkmann’s canals. Other cases may demonstrate areas of dense, sclerotic bone as found in an osteoma. For the practicing pathologist, the most important diagnostic distinction is between HO and extra skeletal osteosarcoma (OS). Helpful histologic findings of HO include presence of bone “maturation” and spatial zonation with more peripherally mature bony elements. As HO matures, woven bone gives way to lamellar bone, which over time is remodeled to develop a cortical appearance. Bone marrow components are recruited into intervening bony elements of HO and gives rise to the normal elements of bone marrow, including a multi lineage marrow, adipocytes, and osteoclasts. As bone maturation occurs, so too does the vasculature within HO develop and mature. Capillary‐like vessels in early HO lesions, also found in granulation tissue‐like areas of fractures, give rise to bone marrow sinusoid type vessels in later HO [9].
The “mature” bony lesions of HO look essentially identical irrespective of their tissue location of origin and are essentially indistinguishable from normal bony elements [10].
Abbreviations
HO – heterotopic ossification
OS – osteosarcoma
References
1. Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, Sono T, McCarthy E, Levi B, James AW. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019; 3(4): e10172. DOI: 10.1002/jbm4.10172.
2. Bedi A, Zbeda RM, Bueno VF, Downie B, Dolan M, Kelly BT. The Incidence of Heterotopic Ossification After Hip Arthroscopy. Am J Sports Med 2012; 40(4): 854–863.
3. Comeau-Gauthier M, Zura RD, Bzovsky S, Schemitsch EH, Axelrod D, Avram V, Manjoo A, Poolman RW, Frihagen F, Heels-Ansdell D, Bhandari M, Sprague S; the HEALTH Investigators. Heterotopic Ossification Following Arthroplasty for Femoral Neck Fracture. J Bone Joint Surg Am 2021; 103(14): 1328–1334.
4. Spinarelli A, Patella V, Petrera M, Abate A, Pesce V, Patella S. Heterotopic Ossification After Total Hip Arthroplasty: Our Experience. Musculoskelet Surg 2011; 95(1): 1–5.
5. Nuovo MA, Norman A, Chumas J, Ackerman LV. Myositis Ossificans with Atypical Clinical, Radiographic, or Pathologic Findings: A Review of 23 Cases. Skeletal Radiol 1992; 21(2): 87–101.
6. Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic Ossification Revisited. Orthopedics 2011; 34(3): 177.
7. Hoch B, Montag A. Reactive Bone Lesions Mimicking Neoplasms. Semin Diagn Pathol 2011; 28(1): 102–112.
8. Hoda SA. Enzinger and Weiss’s Soft Tissue Tumors, 6th Edition. Adv Anat Pathol 2014; 21: 216.
9. Cocks M, Mohan A, Meyers CA, Ding C, LeviB, McCarthy E, James AW. Vascular Patterning in Human Heterotopic Ossification. Hum Pathol 2017; 63: 165–170.
10. Richards PJ, Braid JC, Carmont MR, Maffulli N. Achilles Tendon Ossification: Pathology, Imaging and Aetiology. Disability and Rehabilitation 2008; 30(20–22): 1651–1665.