Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2023, vol. 22(4), pp. 213–219 DOI: https://doi.org/10.15388/LietChirur.2023.22(4).2
Radiological and Clinical Prognostic Factors of Recurrence of Subacute and Chronic Subdural Hematomas
Milda Paliulytė
Faculty of Medicine, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania
E-mail: milda.paliulyte@gmail.com
Gytis Šustickas
Department of Neurosurgery, Republican Vilnius University Hospital, Vilnius, Lithuania
Faculty of Medicine, Utena University of Applied Sciences, Utena, Lithuania
E-mail: gytis.sustickas@gmail.com
Abstract. Objectives. To assess characteristic data of patients hospitalized due to subacute subdural hematomas (SSDHs) and chronic subdural hematomas (CSDHs) and to evaluate radiological findings and establish predictors of hematoma recurrence of SSDHs and CSDHs patient populations. Methods. 149 patients with SSDHs and CSDHs who underwent surgery at Kaunas Clinics Neurosurgery Department from 2020 to 2021 were analyzed. Based on recurrence rate, patients were divided into different subtypes based on computer tomography (CT) imaging. Descriptive analysis, hypothesis testing and correlation matrix were performed using Excel spreadsheet and R programming language with the significance at p-value < 0.05. Results. Out of 149 patients, SSDHs and CSDHs were observed in 89 males (59.6%) and 60 (40.3%) females. Mean of the patients age was 71.1±15 years. Dichotomizing results based on recurrence (cut-off value of 25%), 2 groups were made: 1) low reoperation rate –hypodense sedimented (10%), isodense (21.6%), hypodense (22.2%); 2) high reoperation rate – hypodense bridging (26.7%), hypodense trabecular (27.8%), hypodense with acute bleeding (28.6%), hypodense laminar (30%), isodense with acute bleeding (33.3%). Conclusion. It is concluded that based on 25% reoperation rate high-recurrence and low-recurrence chronic subdural hematomas groups were similar in all terms apart from the hematoma thickness (mm), which leads to a fact that radiological appearance of higher recurrence hematomas should be carefully taken into consideration.
Keywords: chronic subdural hematoma, classification, CT imaging, hematoma thickness, independent predictors, internal architecture, midline shift, recurrence.
Received: 2023/04/27. Accepted: 2023/06/25.
Copyright © 2023 Milda Paliulytė, Gytis Šustickas. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
CSDH being a distinct type of intracranial haemorrhage is one of the most commonly encountered diseases in neurosurgery. In the last decade, it reaches around 8 to 18 cases per 100,000 people per year in older than 65 years generation. In the population aged 80 and more it reaches 36.6/100,000 patients per year. It is about to increase furthermore not only due to demographical changes towards elderly in the population, but also due to increased antithrombotic medication use and other comorbidities under the presentation with expected doubled number in the upcoming 15 to 25 years [1].
In recent years, it has been postulated that changes in the architecture of the hematomas like membranes and septae are the result of self-repair process which on the other hand might decompensate and be used as a prognostic factor for recurrence [2]. This concept was introduced by Nakaguchi et al. and based on the hematomas’ healing stage they could be allocated to one of the four hematoma types with a higher recurrence rate in less organised and most likely younger hematomas like homogenous hypodense, homogenous isodense and sedimented ones [3]. This classification system has already been adopted in a scoring system for recurrence risk assessment [1]. Therefore, recognising factors associated with recurrence rate is a key for treatment options and optimising pre-operative and post-operative management in a timely manner, depending on the local algorithms and protocols [4]. The aim of this study was to identify independent clinical and radiological predictors and their association with recurrence and functional outcome of SSDH and CSDHs in Kaunas Clinics Neurosurgery Department from 2020 to 2021.
Methods
A retrospective cohort investigation of associated factors was performed in 149 patients (89 males and 60 females) with SSDHs and CSDH in Neurosurgery Department in Kaunas Clinics from 2020 to 2021. Patients were selected after assessing hospital records and CT radiological investigations. ICD-10 classification code S06.5 was used as a search term.
Each hematoma was classified according to one of four Nakaguchi subtypes (homogenous, laminar, separated, trabecular type) as described in the original publication [2]. Additionally, The Nakaguchi classification was extended to accommodate all encountered hematomas. Eight distinct subtypes based on hematoma density and internal hematoma architecture as seen in CT imaging in Figure 1 were defined.
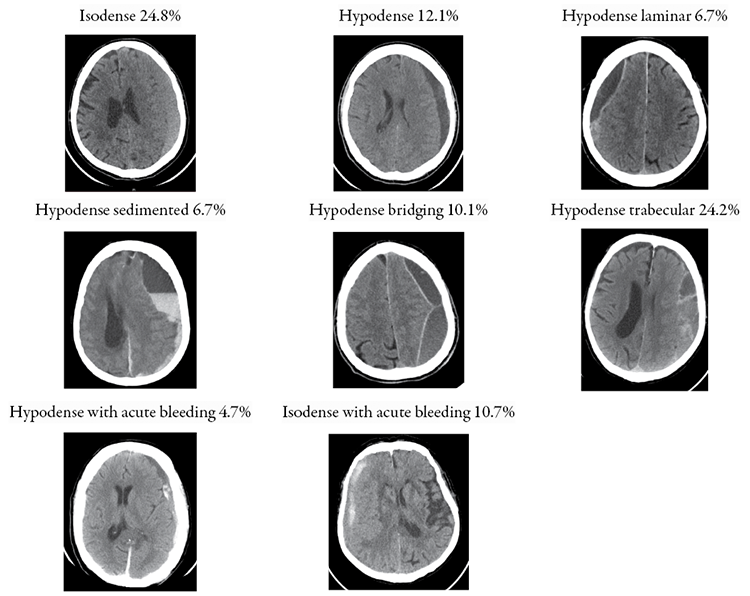
Figure 1. Different subtypes of SSDH and CSDH based on CT imaging
Description of each is as follows: 1) homogenous isodense hematomas: those that are isodense compared to brain tissue; (2) homogenous hypodense hematomas: those that exhibit low density compared to the brain tissue; (3) hypodense laminar: a thin hyperdense visceral or parietal layer (membrane) is visible; (4) hypodense sedimented: different densities within a single hematoma cavity with a clear sedimentation border; (5) hypodense bridging: countable number of internal membranes that connect the visceral and parietal membranes; (6) hypodense trabecular type: uncountable and more complex diffuse inhomogeneous high-density septae between visceral and parietal membranes; (7) hypodense with acute bleeding: chronic subdural hematoma with small hyperdense acute blood components with or without visceral/parietal membranes; (8) isodense with acute bleeding: subacute subdural hematoma with small hyperdense acute blood components with or without visceral/parietal membranes.
All statistical analyses were performed using Excel spreadsheet and R programming language (version 3.4.3; R Foundation for Statistical Computing, Vienna, Austria). Significance was determined at p-value < 0.05.
Data reporting
The study group of 149 patients who had SSDHs or CSDHs included 89 males (59.6%) and 60 (40.3%) females. Patient age was between 32 and 100 with a mean of 71.1±15 years. The medical history of patients included daily alcohol consumption in 21 (14.1%) patients. Initial GCS upon the admission was evaluated from minimum of 3 points to maximum of 15 points with a mean of 13.8 points ±2.3. Out of all 149 patients 103 patients (69.1%) didn’t have coagulopathy disorders whilst 46 patients (30.9%) did have.
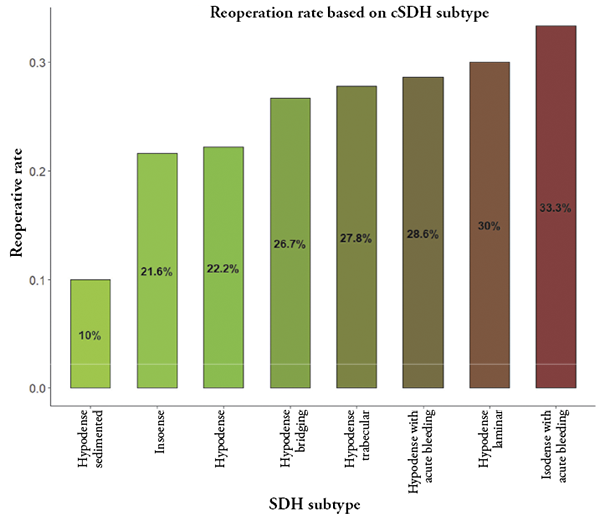
Figure 2. Reoperation rate (%) for each subacute and chronic subdural hematoma type
In this study, subdural hematoma recurrence was observed in 37 (25%) out of all the patients. As initial surgery 144 (97%) patients had burr-hole craniostomy, 4 patients (2.7%) had osteoplastic craniotomy, 37 patients (25%) went through the reoperation and 10 patients (6.8%) had conversion from burr-hole craniostomy to osteoplastic craniotomy. Out of all neurosurgical procedures including burr-hole craniostomy, osteoplastic craniotomy, lumbar drainage, intracranial pressure monitoring device, contaminated would revision 110 (73.8%) patients had 1 operation, 26 patients (17.4%) had 2 operations, 5 patients had 3 operations (3.4%), 5 patients had 4 operations (3.4%), 2 patients had 5 operations (1.3%) and 1 patient had even 8 operations (0.7%) performed. On average patients spent 16.1±11.3 days in the hospital with minimum as little as 1 day and maximum of 67 days. Lethal outcome (mortality) was recorded in 14 patients (9.4%). Numerically dichotomising mRs into two groups 73 patients (49%) had mRS 0–1 (good outcome) and 76 patients (51%) had mRS 2–6 (poor outcome).
In all the cases regardless of SSDHs’ and CSDHs’ radiological appearance, there was 25% incidence of reoperation for the whole cohort. Reoperation rate for each subtype is depicted in Figure 2.
Table 1. Comparison of baseline characteristics between SSDHs and CSDHs of low and high recurrence risk
|
Characteristic |
Low recurrence risk, |
High recurrence risk, Number of patients = 881 |
p-value2 |
|
Midline shift (mm), mean (SD) |
6.2 (5.2) |
7.3 (5.0) |
0.2 |
|
Hematoma thickness (mm), mean (SD) |
18 (8) |
21 (8) |
0.002 |
|
Gender, % |
25 (17%) |
35 (23%) |
0.7 |
|
Age, mean (SD) |
69 (16) |
73 (14) |
0.2 |
|
Alcohol, % |
11 (7.4%) |
10 (6.7%) |
0.4 |
|
Coagulopathy, % |
21 (14%) |
25 (17%) |
0.7 |
|
GCS, mean (SD) |
13.97 (2.05) |
13.70 (2.53) |
0.9 |
|
CRP before surgery, mean (SD) |
29 (46) |
40 (61) |
0.4 |
|
Na (mmol/l) before surgery, mean (SD) |
135.8 (5.6) |
135.8 (4.6) |
0.5 |
|
Na (mmol/l) after surgery, mean (SD) |
137 (6) |
139 (6) |
0.6 |
|
Hb (g/l) before surgery, mean (SD) |
128 (20) |
129 (22) |
0.8 |
|
Hb (g/l) after surgery, mean (SD) |
115 (21) |
114 (23) |
0.7 |
|
WBC (x10^9/l) before surgery, mean (SD) |
10.1 (6.6) |
13.2 (28.7) |
0.2 |
|
PLT (x10^9/l) before surgery, mean (SD) |
246 (103) |
229 (90) |
0.7 |
|
PLT (x10^9/l) after surgery, mean (SD) |
221 (95) |
205 (102) |
0.6 |
|
SPA (%) before surgery, mean (SD) |
73 (25) |
80 (21) |
0.089 |
|
SPA (%) after surgery, mean (SD) |
57 (19) |
55 (25) |
>0.9 |
|
INR before surgery, mean (SD) |
1.41 (1.06) |
1.18 (0.33) |
0.093 |
|
INR after surgery, mean (SD) |
1.41 (0.34) |
1.33 (0.26) |
0.6 |
|
Hospital stay (days), mean (SD) |
16.71 (8.5) |
15.70 (7.2) |
0.59 |
|
Acquired hospital infection, % |
9 (6%) |
16 (11%) |
0.4 |
|
Mortality, % |
8 (5.4%) |
6 (4%) |
0.3 |
|
GOS 1–3 vs 4–5, % |
24 (16%) |
31 (21%) |
>0.9 |
|
mRS 3–6 vs 0–2, % |
34 (23%) |
42 (28%) |
0.8 |
|
1 Mean (SD), %. |
|||
|
2 Wilcoxon rank sum test, Welch Two Sample t-test, Pearson’s Chi-squared test. |
|||
GCS – Glasgow Coma Scale, CRP – C-reactive protein, Na – Sodium, Hb – Haemoglobin, WBC – White blood cells, PLT – Platelet, SPA – Prothrombin activity, INR – International normalised ratio, GOS – Glasgow Outcome Scale, MRS – Modified Rankin Scale.
The described subdural hematomas were dichotomized based on recurrence (cut-off value of 25% reoperation rate) into 2 groups: 1) low reoperation rate – hypodense sedimented, isodense, hypodense; 2) high reoperation rate – hypodense bridging, hypodense trabecular, hypodense with acute bleeding, hypodense laminar, isodense with acute bleeding. The comparison of descriptive characteristics of patients between subdural hematomas of low reoperation risk and high reoperation risk are shown in Table 1.
Discussion. Reoperation rate and radiological findings of different subdural hematomas
Dichotomizing results based on the recurrence (cut-off value of 25%) 2 groups were made: 1) low reoperation rate group: hypodense sedimented (10%), isodense (21.6%), hypodense (22.2%); 2) high reoperation rate group: hypodense bridging (26.7%), hypodense trabecular (27.8%), hypodense with acute bleeding (28.6%), hypodense laminar (30%), isodense with acute bleeding (33.3%). Based on radiological criteria for reoperation, the high-risk patient group had higher hematoma thickness on average (mean of 17.76 [8.06] mm versus 21.32 [8.07] mm, p-value 0.009). Other findings like midline shift (mm), gender, age, alcohol consumption, coagulopathy markers, pre- and post-surgery laboratory results, length of hospitalization, percentage of hospital acquired infections, mortality rate and functional outcome measured in GOS and mRS did not differ between the groups. It is concluded that groups were similar in all terms apart from the hematoma thickness (mm), which leads to a fact that radiological appearance of higher recurrence hematomas should be carefully taken into consideration.
There were discrepancies in the previous studies about the correlation between the recurrence rate and radiological type of CSDHs. Ishita et al. in their study made similar conclusions and stated that hematomas with laminar and sedimented architecture, as well as hyperdense and mixed density hematomas, were associated with the highest likelihood of CSDH recurrence [4]. According to Jack et al., CT scan evidence of septation in a CSDH’s patient increased the likelihood of a recurrence necessitating additional surgery [5]. In addition to that, Han MH et al. al so discovered that hematomas with a homogenous or trabecular internal architecture had a considerably lower recurrence rate than those with a laminar or divided architecture [6]. Furthermore, Stanišic et al. observed that three of the CT features of CSDH assessed were predictive for the postoperative recurrence: the volume of the haematoma before surgery; the isodense, hyperdense, laminar, and separated CT densities; and the volume of the haematoma cavity after drainage has been removed on postoperative day 1 [7]. However, several separate studies have found that laminar hematomas do not have a significant recurrence rate [8, 9], while trabecular hematomas, which correspond to hematomas with numerous cavities, do [10, 11].
Conclusions
1. In this study the average age of patients who had SSDH or CSDH was 71.1±15 years with the majority of males (59.6%). Initial GCS upon the admission was from minimum of 4 points to maximum of 15 points with a mean of 13.8 points ±2.3, 46 patients (30.9%) had some sort of a coagulopathy disorder. On average patients spent 16.1±11.3 days in the hospital ranging from 1 day to 67 days. Mortality was recorded in 14 patients (9.4%) and 76 patients (51%) had a poor outcome (mRS 2–6).
2. In this study overall hematoma recurrence rate was 25% and was associated with these radiological subtypes of hematomas: hypodense bridging (26.7%), hypodense trabecular (27.8%), hypodense with acute bleeding (28.6%), hypodense laminar (30%), isodense with acute bleeding (33.3%).
References
1. Hamou H, Alzaiyani M, Pjontek R, Kremer B, Albanna W, Ridwan H, Clusmann H, Hoellig A, Veldeman M. Risk Factors of Recurrence in Chronic Subdural Hematoma and a Proposed Extended Classification of Internal Architecture as a Predictor of Recurrence. Neurosurg Rev 2022; 45(4): 2777–2786.
2. Nakaguchi HT, Yoshimasu N. Factors in the Natural History of Chronic Subdural Hematomas that Influence their Postoperative Recurrence. J Neurosurg 2001; 95(2): 256–262.
3. Dos Santos RG, Xander PAW, Rodrigues LHDS, Costa GHFD, Veiga JCE, Aguiar GB. Analysis of Predisposing Factors for Chronic Subdural Hematoma Recurrence. Rev Assoc Med Bras 2019; 65(6): 834–838.
4. Miah IP, Tank Y, Rosendaal FR, Peul WC, Dammers R, Lingsma HF, den Hertog HM, Jellema K, van der Gaag NA; Dutch Chronic Subdural Hematoma Research Group. Radiological Prognostic Factors of Chronic Subdural Hematoma Recurrence: A Systematic Review and Meta-Analysis. Neuroradiology 2021; 63(1): 27–40.
5. Liu LX, Cao XD, Ren YM, Zhou LX, Yang CH. Risk Factors for Recurrence of Chronic Subdural Hematoma: A Single Center Experience. World Neurosurg 2019; 132: e506–e13.
6. Han MH, RyuJI, Kim CH, Kim JM, Cheong JH, Yi HJ. Predictive Factors for Recurrence and Clinical Outcomes in Patients with Chronic Subdural Hematoma. J Neurosurg 2017; 127(5): 1117–1125.
7. Schucht P, Fischer U, Fung C, Bernasconi C, Fichtner J, Vulcu S, Schöni D, Nowacki A, Wanderer S, Eisenring C, Krähenbühl AK, Mattle HP, Arnold M, Söll N, Tochtermann L, Z’Graggen W, Jünger ST, Gralla J, Mordasini P, Dahlweid FM, Raabe A, Beck J. Follow-up Computed Tomography After Evacuation of Chronic Subdural Hematoma. N Engl J Med 2019; 380(12): 1186–1187.
8. Bartek J Jr, Sjåvik K, Kristiansson H, Ståhl F, Fornebo I, Förander P, Jakola AS. Predictors of Recurrence and Complications After Chronic Subdural Hematoma Surgery: A Population-Based Study. World Neurosurg 2017; 106: 609–614.
9. Shen J, Yuan L, Ge R, Wang Q, Zhou W, Jiang XC, Shao X. Clinical and Radiological Factors Predicting Recurrence of Chronic Subdural Hematoma: A Retrospective Cohort Study. Injury 2019; 50(10): 1634–1640.
10. Soleman J, Nocera F, Mariani L. The Conservative and Pharmacological Management of Chronic Subdural Haematoma. Swiss Med Wkly 2017; 147: w14398.
11. Lutz K, Kamenova M, Schaedelin S, Guzman R, Mariani L, Fandino J, Soleman J. Time to and Possible Risk Factors for Recurrence after Burr-hole Drainage of Chronic Subdural Hematoma: A Subanalysis of the cSDH-Drain Randomized Controlled Trial. World Neurosurg 2019; 132: e283–e289.
12. Phaphuangwittayakul A, Guo Y, Ying F, Dawod AY, Angkurawaranon S, Angkurawaranon C. An Optimal Deep Learning Framework for Multi-type Hemorrhagic Lesions Detection and Quantification in Head CT Images for Traumatic Brain Injury. Appl Intell (Dordr) 2022; 52(7): 7320–7338.
13. De Bonis P, Olei S, Mongardi L, Cavallo MA, Santantonio M, Trevisi G, Anile C, Mangiola A. Chronic Subdural Hematoma in Patients Aged 80 Years and Older: A Two-Centre Study. Clin Neurol Neurosurg 2018; 170: 88–92.
14. Ou Y, Dong J, Wu L, Xu L, Wang L, Liu B, Li J, Liu W. A Comparative Study of Chronic Subdural Hematoma in Three Age Ranges: Below 40 Years, 41–79 Years, and 80 Years and Older. Clin Neurol Neurosurg 2019; 178: 63–69.
15. Wang C, Liu C. Clinical Characteristics and Surgical Outcomes of Super-Elderly Patients with Chronic Subdural Hematoma. World Neurosurg 2023; 173: e708–e716.
16. Dowlati E, Sarpong K, Triano M, Kamande S, Black J, Mai JC, Anaizi AN, Felbaum DR. Outcomes of Surgical Evacuation of Chronic Subdural Hematoma in the Aged: Institutional Experience and Systematic Review. World Neurosurg 2020; 144: 270–282.
17. Rauhala M, Helén P, Seppä K, Huhtala H, Iverson GL, Niskakangas T, Öhman J, Luoto TM. Long-Term Excess Mortality After Chronic Subdural Hematoma. Acta Neurochir (Wien) 2020; 162(6): 1467–1478.
18. Zanaty M, Park BJ, Seaman SC, Cliffton WE, Woodiwiss T, Piscopo A, Howard MA, Abode-Iyamah K. Predicting Chronic Subdural Hematoma Recurrence and Stroke Outcomes While Withholding Antiplatelet and Anticoagulant Agents. Front Neurol 2020; 10: 1401.
19. Kim SU, Lee DH, Kim YI, Yang SH, Sung JH, Cho CB. Predictive Factors for Recurrence After Burr-Hole Craniostomy of Chronic Subdural Hematoma. J Korean Neurosurg Soc 2017; 60(6): 701–709.
20. Whaley CC, Young MM, Gaynor BG. Very High Blood Alcohol Concentration and Fatal Hemorrhage in Acute Subdural Hematoma. World Neurosurg 2019; 130: 454–458.
21. Christopher E, Poon MTC, Glancz LJ, Hutchinson PJ, Kolias AG, Brennan PM. Outcomes Following Surgery in Subgroups of Comatose and Very Elderly Patients with Chronic Subdural Hematoma. Neurosurg Rev 2019; 42(2): 427–431.
22. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence Based Diagnosis and Management of Chronic Subdural Hematoma: A Review of the Literature. J Clin Neurosci 2018; 50: 7–15.