Acta medica Lituanica ISSN 1392-0138 eISSN 2029-4174
2021. Online ahead of print DOI: https://doi.org/10.15388/Amed.2021.28.1.18
Sensory Perception in Lumbosacral Radiculopathy with Radicular Pain: Feasibility Study of Multimodal Bedside-Suitable Somatosensory Testing
Alfredas Vaitkus*
Clinic of Anaesthesiology and Intensive Care, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
Centre of Anaesthesiology, Intensive Therapy and Pain Management, Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania
ORCID: https://orcid.org/0000-0001-5869-7726
Jūratė Šipylaitė
Clinic of Anaesthesiology and Intensive Care, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
Centre of Anaesthesiology, Intensive Therapy and Pain Management, Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania
ORCID: https://orcid.org/0000-0001-7099-1741
Abstract. Background. Somatosensory testing could be useful in stratifying pain patients and improving pain treatment guidelines. Bedside-suitable techniques are searched for application in daily clinical practice. This study aimed to characterize chronic unilateral lumbosacral radiculopathy (LSR) patients with radicular pain using multimodal bedside-suitable somatosensory testing.
Materials and methods. We evaluated 50 chronic unilateral LSR patients with radicular pain (LSR group) and 24 controls (Control group). Sensory testing was performed using a battery of bedside sensory tests (10g monofilament, 200–400 mN brush, Lindblom rollers with controlled 25°C and 40°C temperature, and 40g neurological pin and investigator’s finger pressure). Participants had to rate their sensory perceptions on both legs at multiple test points within L3 to S2 dermatomes. Characteristics of the testing process and sensory disturbances were analyzed.
Results. LSR group showed sensory disturbances in 82% of patients. The Control group showed no sensory disturbances. Sensory testing took longer (p < 0.001) in the LSR group (29.3 ± 6.5 minutes per patient) than in the Control group (20.5 ± 5.2). Nine sensory phenotypes were detected in the LSR group according to individual sensory disturbances within 5 superficial tests.
Conclusions. The applied multimodal bedside-suitable somatosensory testing battery is suitable for sensory evaluation and characterization of LSR patients. Grouping of allied sensory phenotypes revealed some tendencies in pain intensity characteristics.
Keywords: sensory testing, bedside testing, sensory phenotyping, back pain, lumbosacral radiculopathy.
Jutimų suvokimas sergant juosmens ir kryžmens radikulopatija su šakneliniu skausmu: multimodalinio somatosensorinio ištyrimo prie ligonio lovos tinkamumo tyrimas
Santrauka. Įvadas. Somatosensoriniai tyrimai galėtų būti naudingi klasifikuojant skausmą patiriančius pacientus ir gerinant jų gydymo gaires. Ieškoma jutimų tyrimo metodikų, tinkamų atlikti prie ligonio lovos ir pritaikomų kasdienėje klinikinėje praktikoje. Šiuo tyrimu buvo siekiama įvertinti pacientų, sergančių lėtine vienpuse juosmens ir kryžmens radikulopatija su šakneliniu skausmu, jutimų sutrikimus atliekant multimodalinį somatosensorinį ištyrimą prie ligonio lovos.
Priemonės ir metodai. Mes ištyrėme 50 lėtine vienpuse juosmens ir kryžmens radikulopatija su šakneliniu skausmu sergančių pacientų ir 24 kontrolinės grupės tiriamuosius. Jutimų tyrimas buvo atliktas naudojant prie ligonio lovos tinkamų sensorinių testų bateriją (10 g monofilamentą, 200–400 mN šepetėlį, kontroliuojamos 25 °C ir 40 °C temperatūros Lindblomo volelius, 40 g neurologinę adatėlę bei tyrėjo piršto paspaudimą). Tyrimo dalyviai turėjo įvertinti sensorinių dirgiklių sukeliamus pojūčius abiejų kojų simetriniuose taškuose išilgai L3–S2 dermatomų. Atlikta jutimų tyrimo eigos ir nustatytų jutimų sutrikimų analizė.
Rezultatai. Juosmens ir kryžmens radikulopatijos grupės 82 proc. tiriamųjų buvo nustatyti jutimų sutrikimai. Kontrolinės grupės tiriamiesiems jutimo sutrikimų nenustatyta. Vidutinė vieno paciento jutimų tyrimo trukmė visa sensorinių testų baterija juosmens ir kryžmens radikulopatijos grupėje buvo ilgesnė nei kontrolinėje grupėje (29,3 ± 6,5 min vs 20,5 ± 5,2 min; p < 0,001). Juosmens ir kryžmens radikulopatijos grupės tiriamiesiems pagal individualius jutimo sutrikimus penkiuose paviršinių jutimų testuose buvo nustatyti devyni sensoriniai fenotipai.
Išvados. Tyrime naudota multimodalinio somatosensorinio ištyrimo prie ligonio lovos testų baterija yra tinkama jutimams vertinti. Grupuojant giminingus sensorinius fenotipus buvo nustatyta skausmo intensyvumo skirtumų.
Raktiniai žodžiai: sensorinis tyrimas, ištyrimas prie ligonio lovos, sensorinis fenotipavimas, nugaros skausmas, juosmens ir kryžmens radikulopatija.
_______
* Corresponding author: Alfredas Vaitkus, Centre of Anaesthesiology, Intensive Therapy and Pain Management, Vilnius University Hospital Santaros Klinikos, Santariškių g. 2, LT-08661, Vilnius, Lithuania. Phone: +37065260115. E-mail: alfredas.vaitkus@santa.lt
Received: 02/02/2021. Revised: 29/03/2021. Accepted: 12/04/2021
Copyright © 2021 Alfredas Vaitkus, Jūratė Šipylaitė. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Low back pain (LBP) is a leading cause of disability defined by the global all-age years lived with disability (YLDs) index in 2017 [1]. Classic LBP treatments vary from passive to active, from noninterventional to surgical, and from traditional to modern. Still, the question of which method suits each patient best remains open, as evidence is often insufficient to conclude [2]. Treatment response varies significantly among all treatment methods [3]. There is a tendency of phenotyping pain patients to characterize them more accurately and facilitate the selection of available treatments [3]. Quantitative sensory testing (QST) is used to evaluate sensory disturbances, and it is applicable in patients with back pain pathology [4]. Standardized and automated QST is a reliable method supplying detailed characteristics of sensory function. However, its application in daily clinical practice remains limited due to the expensiveness of equipment, considerable consumption of time, and the need for personnel training.
Also, for the application of comprehensive QST in patients with radiculopathy, there is uncertainty regarding site selection for sensory testing. Low back pain with radiculopathy could manifest with the pain of different mechanisms – nociceptive, referred, neuropathic, or a mix of any of these. Pain is usually related to patients’ posture, activity, and some other factors [5]. Therefore, it is difficult to determine the origin and mechanisms at patients’ reported pain sites. QST protocols recommend testing the site of greatest pain [6]. Radicular pain related to lumbosacral radiculopathy (LSR) is often reported along the involved segments or at separate locations of its. If to test several sites using comprehensive QST protocol, it would take plenty of time. This would question its accuracy due to the patient’s fatigue. Therefore, it would be appropriate to screen all LSR related segmental zones in a reasonable amount of time. More simple nonautomated QST approaches and sensory mapping techniques have also been suggested to address this problem [7]. Furthermore, the qualitative sensory testing principle was proposed and tested for the detection of atypical odontalgia [8], stenosis involving the L5 nerve root [9], and for assessment of nerve block quality with local anesthetic [10].
We hypothesized that a bedside-suitable sensory testing battery describing qualitative characteristics could demonstrate the variability of sensory disturbances in LSR patients with radicular pain and could be applicable for sensory phenotyping.
Methods
Study aims and objectives
The presented study is a prospective diagnostic study aimed to explore the feasibility of bedside-suitable multimodal somatosensory testing for the evaluation of LSR patients with radicular pain. The objectives of this study were: to assess the capability of selected testing battery to detect sensory disturbances in LSR patients, also to evaluated the time needed to perform sensory testing and patient cooperation issues. The secondary aim of this study was to characterize typical sensory disturbances in LSR patients with radicular pain. The main study group was collected of chronic painful unilateral LSR patients (LSR group). The Control group consisted of chronic pain patients without LSR but with pain syndromes anatomically unrelated to the body region being explored by sensory testing, i.e., pain syndromes not involving the lower waist and legs. The decision to form the Control group of pain patients (not healthy subjects) was made because sensory testing is a psychophysical testing type. Therefore, subjects free of pain would have a different psychophysical background.
Ethics
The Vilnius Regional Biomedical Research Ethics Committee approved this study protocol (#158200-15-772-289). All participants were informed about the goal and the study process and gave their informed consent to participate.
Participants
The study was carried out from 2015 to 2018. The patients were recruited from those who were referred to the Pain Medicine Centre at the Vilnius University Hospital Santaros Klinikos (Vilnius, Lithuania) on a scheduled basis. During the screening, all consecutive patients meeting inclusion criteria for the LSR or Control group were invited.
The inclusion criteria for the LSR group were chronic painful unilateral LSR patients complaining of leg pain (with or without low back pain) and pain duration of >3 months. Criteria for painful LSR were typical radiating segmental pain with at least one sign of neurological impairment of corresponding spinal segments (positive straight leg raise (SLR) test at an interval of 0–70° or sensory impairment at testing with a standard neurological pin or motor function impairment less than 5th-grade muscle strength according to Lovett’s scale or impairment of deep tendon reflexes). The results of the radiological examination had to be compatible with clinical symptoms.
The Control group was formed of chronic pain patients (pain duration of >3 months) without LSR and with pain syndromes anatomically unrelated to the lower waist and legs. The age limits for both groups were 18 to 85 years.
Exclusion criteria for both groups are presented in Table 1.
Table 1. Exclusion criteria.
|
1. Exclusion criteria for both LSR and control groups:
|
|
2. Additional exclusion criteria for the LSR group:
|
LSR – lumbosacral radiculopathy; CNS – central nervous system; NRS – numeric rating scale
Pain evaluation consisted of: pain intensity evaluated separately for low back and leg (Brief Pain Inventory short form (BPI-SF) Pain items, numeric rating scale 0 to 10 points (NRS 0-10)), pain impact on functioning (BPI-SF Interference items, NRS 0-10), anxiety and depression evaluation (Hospital Anxiety and Depression Scale (HADS) score), screening for neuropathic pain component (painDETECT Questionnaire (PD-Q) score). Assessment of magnetic resonance imaging (MRI) and/or computed tomography (CT) of the lumbosacral spine not older than 6 months were collected and analyzed. Sensory testing was performed on all patients, collecting data on sensory disturbances, testing duration, and any test completion problems.
Sensory testing
A single investigator performed sensory testing. Dermatomes L3, L4, L5, S1, and S2, according to the dermatomal distribution map from Bonica’s Management of Pain [11] were tested at three levels: thigh, calf, and foot. At least 2 points for each level of every dermatome were tested. Hence, at least 6 points for 1 dermatome were tested in one leg, starting proximally and proceeding distally. In every modality each dermatome was tested sequentially one by one. Similar sensory testing was performed in both groups. Sensory function was evaluated by comparing sensory perceptions on both legs. In the LSR group, the involved (painful) leg was compared to the nonpainful leg. Patients were asked to compare and report their sensory perceptions at each pair of tested symmetrical points on both legs (“mirror image” points) at least twice. The quality of sensory perception in every tested point was rated separately as normal sensitivity (if similar to control side), hyposensitivity (reduced sensory perception or no perception), or hypersensitivity (increased sensory perception or painful sensation in cases of nonpainful stimuli). When two tests at any test points were inconsistent, third or more tests were performed with a slight change in the testing point location and comparison to a secondary control point. The anterolateral same-side surface of the trunk at the umbilical level was used as a secondary control site.
If the perception in the radiculopathy-affected leg was rated as being normally sensitive at all points examined, the tested modality was qualified as normal. If the sensory perception in any of the tested dermatomes was rated as hyposensitive or hypersensitive in at least two adjacent test points along the dermatome, then the tested modality was qualified as hyposensitive or hypersensitive, respectively. The dermatomes with sensory disturbances had to correspond to the segments defined in the clinical diagnosis of radiculopathy. Noncompliance with radiculopathy-affected segments was qualified as an exclusion criterion.
The full sensory testing battery consisted of 6 tests (5 superficial and 1 deep sensory perception), as listed in Table 2. All superficial sensory tests were performed using standardized instruments that are approved and manufactured for this purpose.
Table 2. The battery of sensory tests used in the study with corresponding sensory modalities and axons tested. All tests are presented in the order of testing. The order of tests performed and general requirements for testing were followed according to the guidelines set by Walk et al. (2009) [7].
|
Sensory |
Axon type primarily tested [12] |
Instruments and their |
|
Superficial sensory tests |
||
|
Mechanical tactile nonpainful static |
Aβ |
10g monofilament (Neuropen, Owen Mumford Ltd, Woodstock, UK) |
|
Mechanical tactile nonpainful dynamic |
Aβ |
200–400 mN brush (SENSELab Brush-05, Somedic SenseLab AB, Sösdala, Sweden) |
|
Thermal of innocuous cold |
Aδ |
Lindblom roller with a controlled temperature of 25°C (Rolltemp, Somedic SenseLab AB, Sösdala, Sweden) |
|
Thermal of innocuous warmth |
C |
Lindblom roller with a controlled temperature of 40°C (Rolltemp, Somedic SenseLab AB, Sösdala, Sweden) |
|
Mechanical tactile painful, pinprick |
Aδ, C |
40g neurological pin (Neurotip/Neuropen, Owen Mumford Ltd, Woodstock, UK) |
|
Deep tissue sensory test |
||
|
Deep pressure |
A, C |
Finger pressure with moderate force |
Statistics
We used descriptive statistics such as frequency tables and means (with standard deviation) to describe quantitative and qualitative data, respectively. The normality of the distribution was assessed using the Kolmogorov–Smirnov test. Differences between the two independent quantitative normally distributed, nonnormally distributed, and qualitative groups were evaluated by the Student’s t-test, Mann–Whitney U test, and the chi-square test, respectively. A Kruskal–Wallis one-way ANOVA was used to assess the mean differences in more than 2 groups. The Bonferroni correction was used for pairwise comparisons. A two-tailed p-value less than 0.05 was considered significant. Statistical analysis was performed using the statistical analysis software (SAS) package version 9.2.
Results
Study flow
A total of 362 patients were screened for enrollment into the LSR group. 56 patients were enrolled into the LSR group, and 50 of them completed it (Figure 1). Twenty-four patients were enrolled into the Control group and completed the study.
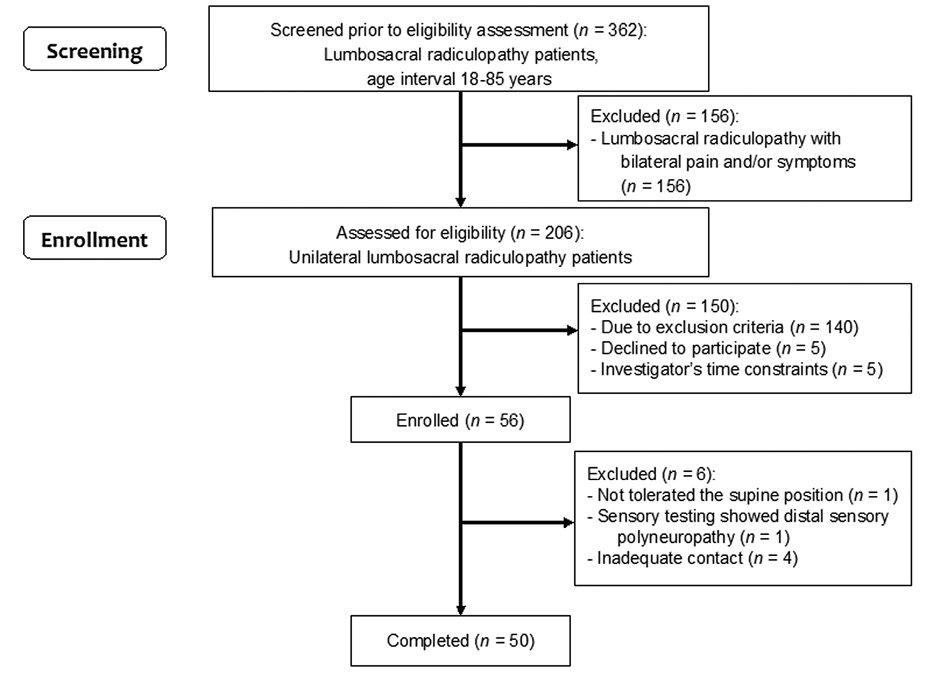
Figure 1. Flowchart for the inclusion of study participants into the LSR group.
General characteristics
Fifty LSR patients (30 women, 20 men; mean age ± SD: 59.5 ± 10.3, range 38–79 years) and 24 control group patients (17 women, 7 men; mean age ± SD: 56.1 ± 12.7, range 30–85 years) completed this study. Post hoc analysis showed both groups to be age- and sex-matched. The groups differed reliably in weight (83.0 vs 73.0 kg, p = 0.017) and body mass index (29.0 vs 26.1, p = 0.001), which is consistent with data confirming association of higher weight with LBP [13].
Pain characteristics
Pain in the leg was present in all LSR patients (52% right side, 48% left side). Low back pain was present in 88% of the LSR patients. Detailed pain and related characteristics of the LSR group are presented in Table 3.
Table 3. Pain and related characteristics in the LSR group (n = 50).
|
Variable |
Summary statistic |
|
Pain duration – Low back (months): Overall Current intensity |
61 (± 61), 0–255 5 (± 5), 0–26 |
|
Pain duration – Leg (months): Overall Current intensity |
29 (± 34), 3–180 5 (± 4), 0–16 |
|
Pain intensity – Low back (BPI-SF, NRS 0-10): Worst during last week Least during last week Average during last week Current pain |
7.0 (± 2.6), 0–10 2.0 (± 2.1), 0–6 4.9 (± 2.1), 0–8 4.2 (± 2.8), 0–10 |
|
Pain intensity – Leg (BPI-SF, NRS 0-10): Worst during last week Least during last week Average during last week Current pain |
7.4 (± 1.5), 4–10 1.8 (± 2.5), 0–8 5.3 (± 2.0), 2–10 3.7 (± 3.2), 0–10 |
|
Impact on functioning (BPI-SF, NRS 0-10): General activity Mood Walking Work Relations Sleep Enjoyment of life Mean of all |
7.5 (± 2.0), 2–10 7.3 (± 2.5), 0–10 7.5 (± 1.9), 2–10 8.2 (± 1.3), 5–10 5.2 (± 3.2), 0–10 6.1 (± 3.1), 0–10 7.2 (± 2.7), 0–10 7.0 (± 1.8), 3.3–10 |
|
States of anxiety and depression (HADS score): Anxiety Depression |
8.0 (± 4.7), 1–18 5.8 (± 2.1), 1–11 |
|
Neuropathic pain component (PD-Q score) |
9.5 (± 3.8), 2–20 |
LSR – lumbosacral radiculopathy; SD – standart deviation; BPI-SF – Brief Pain Inventory short form; NRS – numeric rating scale; HADS – Hospital Anxiety and Depression Scale; PD-Q – painDETECT Questionnaire.
Other complaints concomitant to pain were reported: paresthesia/numbness – 72%, muscle cramps – 22%, feeling of cold in the leg – 12%, and leg swelling – 4%.
Pain characteristics in the Control group were: pain duration 43.6 ± 70.2 months, range 3–312, pain intensity as average during last week 5.3 ± 1.16 points on NRS 0-10, range 3–7. These variables differed from the LSR group with statistical significance of p = 0.029 and p = 0.023, respectively, but this was treated as indeterminant for comparative sensory testing results.
Neurological evaluation
The distribution of nerve root impairment according to LSR diagnosis is shown in Figure 2. L5 root was the most often involved in the diagnosis, 80% of the cases, S1 in 68%, L4 in 48%, and L3 in 4% of cases.
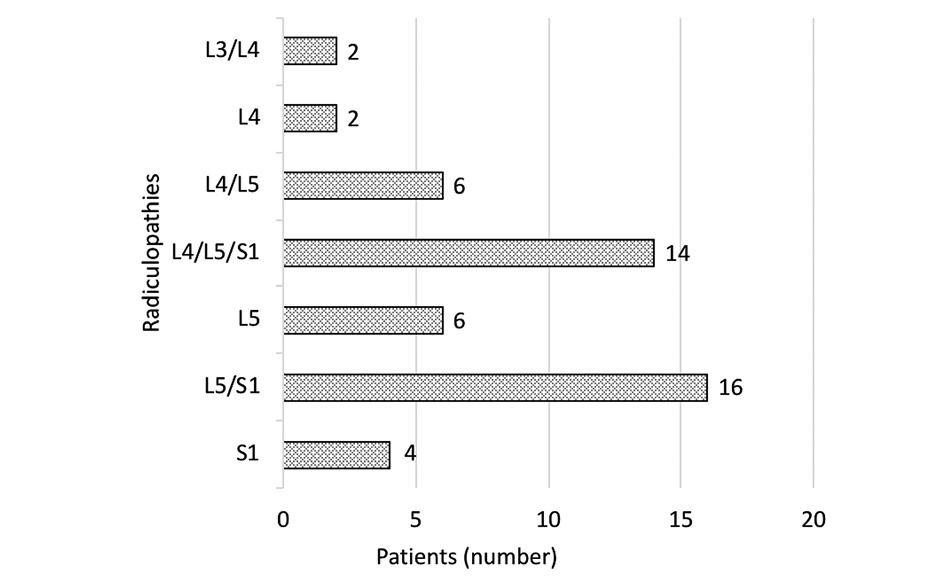
Figure 2. Nerve root impairment according to diagnosis in the LSR group (n = 50).
Sensory impairment by testing with a standard neurological pin was detected in 76% of patients, impairment of deep tendon reflexes was detected in 64% of patients, and motor function impairment was detected in 72% of patients. Positive SLR test was detected in 88% of patients with 39° ± 16° (range 15°–70°). Eight percent of patients without any neurologic deficit were diagnosed with LSR based on typical segmental radicular pain distribution with a positive SLR test present. Neurogenic claudication was reported by 38% of patients.
Radiographic characteristics
The sole selective radiologic finding corresponding to spinal nerve involvement of LSR diagnosis was detected in 54% of cases. All other patients (46%) could be defined as having diffuse/multiple degenerative changes compatible with LSR diagnosis, though it is difficult to determine the exact anatomical site of spinal nerve impingement.
Sensory characteristics
Sensory testing took significantly longer in the LSR group (29.3 ± 6.5 minutes per patient) than in the Control group (20.5 ± 5.2 minutes per patient), p < 0.001.
Patients from the Control group showed no sensory impairment or asymmetry between the left and right legs to all tested sensory modalities.
In the LSR group, 82% of the patients showed sensory disturbances. The full spectrum of disturbances is presented in Figure 3. A single type of disturbance inside each separate sensory modality (hypersensitivity or hyposensitivity) was registered per patient except in deep pressure testing. In deep pressure testing, 16% of cases showed mixed hypo- and hypersensitivity (at different testing points).
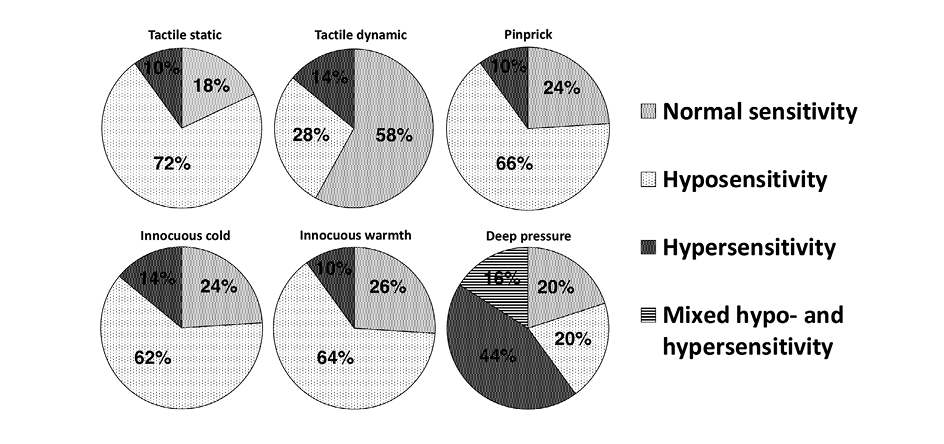
Figure 3. Proportions of patients with sensory disturbances detected at different modalities in the LSR group (n = 50).
The frequency of hyposensitivity to the tactile dynamic test was much rarer compared to other types of superficial sensory tests. Statistical comparison of proportions showed that the percentage frequency of hyposensitivity and other sensory types (normal sensitivity and hypersensitivity) differed significantly in tactile dynamic testing (28% and 72%) if to compare to tactile static testing (72% and 28%, p < 0.001), to pinprick testing (66% and 34%, p < 0.001), to cold testing (62% and 38%, p = 0.001), and to warmth testing (64% and 36%, p < 0.001).
Grouping LSR patients by disturbances in each superficial sensory test revealed nine sensory phenotypes (Table 4).
Table 4. Sensory phenotypes according to superficial sensory testing in the LSR group (n = 50).
|
Pheno-types |
Number of patients |
Sensory disturbancesa |
Sensory group |
||||
|
Tactile static |
Tactile dynamic |
Pinprick |
Innocuous cold |
Innocuous warmth |
|||
|
I |
9 |
Normal |
Normal |
Normal |
Normal |
Normal |
Normal sensitivity group |
|
II |
2 |
Hypo |
Normal |
Normal |
Normal |
Normal |
Hyposensitivity to 1 to 3 modalities group |
|
III |
1 |
Hypo |
Normal |
Normal |
Normal |
Hypo |
|
|
IV |
2 |
Hypo |
Normal |
Hypo |
Hypo |
Normal |
|
|
V |
15 |
Hypo |
Normal |
Hypo |
Hypo |
Hypo |
Hyposensitivity to 4 to 5 modalities group |
|
VI |
12 |
Hypo |
Hypo |
Hypo |
Hypo |
Hypo |
|
|
VII |
2 |
Hypo |
Hyper |
Hypo |
Hypo |
Hypo |
|
|
VIII |
2 |
Hypo |
Hypo |
Hypo |
Hyper |
Hypo |
|
|
IX |
5 |
Hyper |
Hyper |
Hyper |
Hyper |
Hyper |
Hypersensitivity group |
a Meanings: Normal – normal sensitivity, Hypo – hyposensitivity, Hyper – hypersensitivity.
The sensitivity of each superficial sensory test in the detection of LSR was as follows: tactile static test – 82%, pinprick – 76%, innocuous cold – 76%, innocuous warmth – 74%, and tactile dynamic – 42%. There were no superficial sensory test combinations that increased single tactile static testing sensitivity in detecting lumbosacral radiculopathy.
Correlations of sensory disturbances in the LSR group with other patient characteristics (demographic, pain, radiologic spine changes) have been tested. As patient groups in different phenotypes were small, the following grouping of allied phenotypes has been applied. The following groups were formed (Table 4): “Normal” (phenotype I patients with normal sensitivity in all tested modalities), “1-3 hypo” (phenotype II–IV patients with relatively less hyposensitivity – in 1, 2, or 3 modalities), “4-5 hypo” (phenotype V–VIII patients with relatively more hyposensitivity – in 4 or 5 modalities) and “Hyper” (phenotype IX patients with hypersensitivity in all tested modalities). Though statistically significant differences were not detected, the “Normal” patient group tended to show higher pain intensities than other groups in all low back and leg pain categories. The “Hyper” patient group tended to show lower pain intensities in most pain categories (Figure 4). Larger patient groups are needed to determine whether correlations are statistically significant. A full list of distribution of characteristics across groups of sensory phenotypes is presented in Table 5.
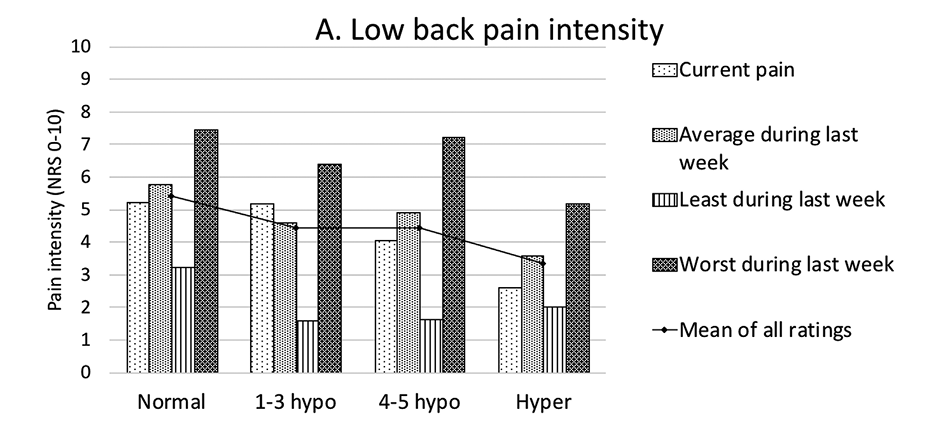
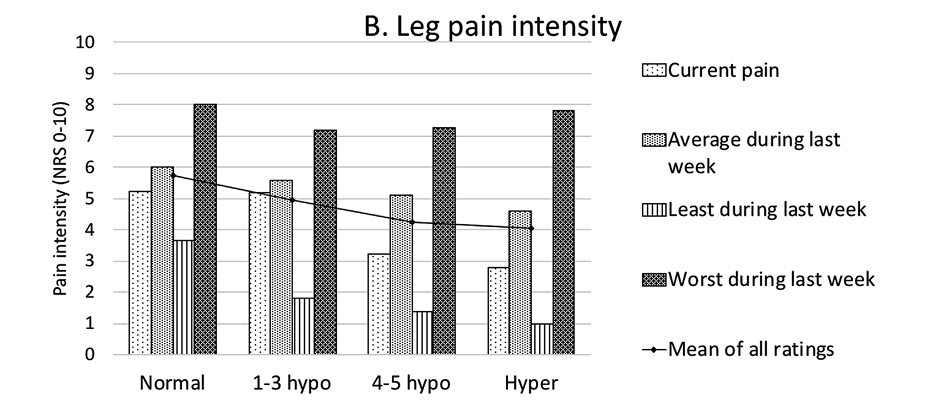
Figure 4. Low back (A) and leg (B) pain intensities differed between groups of sensory phenotypes. Pain intensities are presented as means in points on NRS 0-10. Differences between groups are insignificant. NRS – numeric rating scale.
Table 5. Analysis of characteristics between groups of sensory phenotypes in the LSR group. Differences between groups are insignificant.
|
Variable |
Groups of sensory phenotypes |
|||
|
Normala |
1-3 hypob |
4-5 hypoc |
Hyper d |
|
|
Gender (n (%)): |
||||
|
Female Male |
6 (66.7) 3 (33.3) |
4 (80.0) 1 (20.0) |
19 (61.3) 12 (38.7) |
1 (20.0) 4 (80.0) |
|
Age (years, mean (± SD)) |
67.6 (± 9.3) |
54.4 (± 6.5) |
58.8 (± 10.1) |
55.0 (± 10.7) |
|
Body mass index (kg/m2, mean (±SD)) |
30.2 (± 3.6) |
27.4 (± 5.1) |
29.0 (± 4.2) |
28.0 (± 4.1) |
|
Straight leg raise (degrees, mean (±SD)) |
49 (± 13) |
48 (± 13) |
35 (± 13) |
34 (± 14) |
|
Pain duration – Low back (months, mean (±SD)): |
||||
|
Overall Current intensity |
50 (± 41) 3 (± 2) |
48 (± 63) 3 (± 4) |
65 (± 61) 6 (± 5) |
68 (± 99) 7 (± 11) |
|
Pain duration – Leg (months, mean (±SD)): |
||||
|
Overall Current intensity |
31 (± 37) 3 (± 1) |
37 (± 32) 3 (± 4) |
28 (± 37) 5 (± 4) |
20 (± 18) 5 (± 4) |
|
Impact on functioning (BPI-SF Interference Items, NRS 0-10, |
||||
|
General activity Mood Walking Work Relations Sleep Enjoyment of life Mean of all |
8.2 (± 1.4) 8.3 (± 1.5) 7.7 (± 1.6) 8.4 (± 1.4) 4.4 (± 3.8) 7.2 (± 1.8) 8.5 (± 1.4) 7.6 (± 1.3) |
6.4 (± 4.0) 5.6 (± 3.4) 6.2 (± 3.5) 8.6 (± 0.5) 7.0 (± 1.9) 5.0 (± 3.3) 6.8 (± 4.4) 6.5 (± 2.9) |
7.5 (± 1.6) 7.1 (± 2.5) 7.7 (± 1.5) 8.0 (± 1.4) 5.2 (± 3.1) 5.8 (± 3.4) 6.7 (± 2.7) 6.9 (± 1.7) |
7.6 (± 2.3) 8.0 (± 1.9) 6.6 (± 2.2) 8.2 (± 1.5) 4.4 (± 3.8) 6.6 (± 3.2) 8.0 (± 1.9) 7.1 (± 2.3) |
|
States of anxiety and depression (HADS score, mean (±SD)): |
||||
|
Anxiety Depression |
7.0 (± 3.7) 6.9 (± 0.7) |
12.0 (± 4.5) 6.2 (± 1.3) |
7.5 (± 4.4) 5.5 (± 2.2) |
10.5 (± 5.3) 5.5 (± 3.1) |
|
Neuropathic pain component (PD-Q score, mean (±SD)) |
9.0 (± 3.5) |
9.6 (± 1.5) |
9.6 (± 4.4) |
8.8 (± 2.9) |
a “Normal” group includes patients with normal sensitivity in all tested modalities; b “1-3 hypo” group – patients with detected hyposensitivity in 1, 2, or 3 modalities; c “4-5 hypo” group – patients with detected hyposensitivity in 4 or 5 modalities; d “Hyper” group – patients with detected hypersensitivity in all tested modalities; LSR – lumbosacral radiculopathy; SD – standard deviation; BPI-SF – Brief Pain Inventory short form; NRS – numeric rating scale; HADS – Hospital Anxiety and Depression Scale; PD-Q – painDETECT Questionnaire.
Patient cooperation
Thirteen (9.3%) of 140 excluded patients were categorized with “inability to understand study instructions or documentation properly.” This group consisted of patients with insufficient cognitive function or language knowledge (a peculiarity of the regional multilingual population). Additionally, four (7.1%) of 56 patients enrolled in the trial were excluded due to inconsistent or inconclusive sensory testing.
Discussion
Qualitative type sensory testing is relatively uncommon in clinical trials as comprehensive automated QST still reigns as the gold standard. In contrast to QST, qualitative sensory testing tools create a fixed-intensity stimulus. They allow classification of sensory perception as either normal or not if compared to an intact reference testing site without determining the degree of sensory dysfunction. Like QST, qualitative sensory testing provides information on the perception of stimulation to different types of receptors and their nerve fibers.
The duration of the full testing battery was 29.3 ± 6.5 minutes per patient. Such duration seems quite adequate and corresponds to an intended 30 minutes for at least one test point of the standardized QST protocol by the German Research Network on Neuropathic Pain [14]. During this time, the applied testing permitted screening of multiple (L3-S2) segments and multiple test points along the whole segment length.
Exceptional attention was paid to patient selection (inclusion/exclusion criteria) to avoid any factors that might misrepresent sensory function and testing. Therefore, screening for patients with pure unilateral LSR and excluding any patients with diseases, conditions, or factors that could affect sensations in the areas of the body being studied was critical. A significant list of exclusion criteria considerably reduced the number of patients screened for inclusion, with 140 (68%) of 206 study candidates excluded. This is the primary limiting factor for this type of sensory testing to be used in clinical practice.
Also, good patient cooperation is essential to ensure the effective use of sensory testing. The patient cooperation level in our study was acceptable. The overall withdrawal rate due to inconsistent contact or linguistic issues was 8% (17 patients of 206).
All sensory testing instruments were easy to handle; no technical problems occurred. The price of the whole set of testing instruments was approximately 2000 euros. This is about 10 times less than comprehensive automated QST systems.
In our study, nine (18%) of the 50 subjects did not report any superficial disturbances (12%, if including deep tissue pressure evaluation). Normal sensations were found in 18% to 58% if the different tests were assessed separately. Intact sensory function or a relatively low degree of sensory impairment difficult to distinguish from the unaffected side of the body are among possible explanations. Qualitative type of sensory testing in patient populations with unilateral sciatica [15], in lumbar nerve-root compression syndromes [16], in radicular pain patients [17], or symptomatic lumbar lateral stenosis involving L5 root [9] showed similar frequencies (27% to 55%) of normal sensitivity.
The dominant impairment type detected in our study is hyposensitivity. This conforms with all other radiculopathy studies. Hyposensitivity to most superficial tests (to 4 or 5 tests) dominated across the study population and accounted for 31 (62%) of the 50 study patients.
Patients with hypersensitivity are another specific group. Five of the 50 patients showed hypersensitivity to all superficial tests. Another four patients showed isolated hypersensitivity to one modality (brush or cold perception), with hyposensitive responses to all other tests. While hypersensitivity phenomena were common in one report with qualitative type testing study [18], some other studies with QST report hypersensitivity as a rare finding [17, 19, 20]. Some other radiculopathy studies did not record hypersensitivity or included them in the general group of “sensory impairments” [9]. Sensory hypersensitivity is a marker of nervous system sensitization [21]. Also, hypersensitivity could be related to sympathetic fibers sprouting on dorsal root ganglion cells [22].
Brush testing (200–400 mN) for tactile dynamic perception showed statistically significantly the smallest portion of hyposensitivity cases than other sensory modalities. This resembles findings of sensory testing with cotton swab stroke in atypical odontalgia patients [8].
Deep tissue sensitivity testing was performed with a nonstandardized instrument. The results of this test exhibited a relatively high percentage of hypersensitivity (44% in deep pressure test vs. 10–14% in other modalities) and the presence of mixed hypo- and hypersensitivity together in the same patient at different tested points (16% of patients). The most reliable explanation of such intra-individual mixed type disturbances could be the different underlying tissues (bony prominences, muscles with bony structures underneath at different depths) at various testing sites, which could be of great importance when testing the lower limb [23]. A mechanical pressure algometer was tested during study preparations as a possible instrument for deep tissue sensitivity testing. Maintenance of stable fixed pressure during the test appeared to be technically complex and/or inaccurate. It seemed confusing for patients to report perceived pressure since they had to evaluate only the final pressure achieved and maintained for several seconds. Therefore, finger pressure testing was chosen for testing, as it was recommended for bedside testing in some publications [17, 23]. To improve the set of bedside-suitable tests, a standardized deep tissue pressure testing instrument that would produce and maintain a fixed amount of pressure is needed. Also, a proper level of pressure that would be most informative for the screening of various body regions should be established.
The unexpected finding was the neuropathic scores obtained with PD-Q. In general, PD-Q scores in LSR group were low (9.5 ± 3.8) with 81% of patients qualified as ‘unlikely neuropathic pain’, 17% as ‘uncertain’, and only 2% as ‘likely neuropathic pain’. Given that study population consisted of carefully selected LSR patients, the results appear to be in line with publications on this subject [24].
We did not intend to measure the reliability of testing techniques. The use of nonstandardized testing instruments has already been investigated in atypical odontalgia patients [8] and exhibited sufficiently high intra-rater and inter-rater reliability. Also, some bedside sensory testing tools for detecting of cold, warm, mechanical, and pressure pain thresholds have already been proven to correlate with the respective QST parameters significantly [25]. We used strictly standardized instrumentality for superficial sensory testing. Therefore, it is likely that the reliability rates would not be lower than in the above study.
Data from QST studies usually report sensory function in group averages of quantitative indicators. However, individual variability remains unclear. Our study revealed considerable variability of sensory disturbances – nine sensory phenotypes were distinguished according to individual sensory profiles. This was also reported with QST in cervical radiculopathy [26]. The variety of such sensory phenotypes could reflect the diversity of causative spinal changes in our aged study population and various degrees of root impairment. Potentially, sensory testing data could be related to LSR treatment effects or other prognostic factors. The available data are contradicting. The current perception threshold testing was evaluated in patients with lumbar radiculopathy before and after epidural steroid injection [27] without correlation in pain intensity changes after the treatment. Later studies with QST showed some potential to predict improvement after epidural steroid injection for sciatica-related pain [28] and in predicting functional status after microdiscectomy in patients with LSR [20]. QST could potentially reveal the treatment implicated mechanism of action of spinal manipulative therapy [29]. A review and meta-analysis of QST related prediction of musculoskeletal pain-related outcomes state that QST might help to identify persons to benefit most from treatment interventions [30]. Further studies are needed to address the optimal bedside testing battery issue to detect sensory changes determinant for decision-making.
Conclusion
The applied multimodal bedside-suitable somatosensory testing battery is suitable for sensory evaluation, characterization and phenotyping of LSR patients with radicular pain. Tactile static testing with a 10g monofilament showed the highest sensitivity to detect superficial sensory disturbances. It could be recommended as the most sensitive single testing instrument. Tactile dynamic testing with 200–400 mN brush detected sensory impairments significantly less frequently than any other modalities, therefore not recommended for the screening of LSR patients. The detected sensory phenotypes should be explored for the relation with treatment effects.
Acknowledgment
The authors would like to thank Tadas Žvirblis for carrying out the statistical analysis.
Research funding
No funding was involved.
Conflict of interest
Nothing to declare.
References
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1789–858. doi https://doi.org/10.1016/S0140-6736(18)32279-7
- Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J 2014;14(1):180–91. doi https://doi.org/10.1016/j.spinee.2013.08.003
- Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 2016;157(9):1851–71. doi https://doi.org/10.1097/j.pain.0000000000000602
- Schäfer AG, Hall TM, Rolke R, Treede RD, Lüdtke K, Mallwitz J, et al. Low back related leg pain: an investigation of construct validity of a new classification system. J Back Musculoskelet Rehabil. 2014;27(4):409-418. doi 10.3233/BMR-140461
- Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain 2009;147: 17–19. doi https://doi.org/10.1016/j.pain.2009.08.020
- Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013;154(9):1807-19. doi https://doi.org/10.1016/j.pain.2013.05.047
- Walk D, Sehgal N, Moeller-Bertram T, Edwards RR, Wasan A, Wallace M, et al. Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin J Pain 2009;25(7):632-40. doi https://doi.org/10.1097/AJP.0b013e3181a68c64
- Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, Drangsholt M, et al. Chairside intraoral qualitative somatosensory testing: reliability and comparison between patients with atypical odontalgia and healthy controls. J Orofac Pain 2013;27(2):165-70. doi https://doi.org/10.11607/jop.1062
- Lin JH, Hsieh YC, Chen YC, Wang Y, Chen CC, Chiang YH. Diagnostic accuracy of standardised qualitative sensory test in the detection of lumbar lateral stenosis involving the L5 nerve root. Sci Rep 2017;7(1):10598. doi https://doi.org/10.1038/s41598-017-10641-2
- Kothari SF, Shimosaka M, Iida T, Komiyama O, Shibutani K, Svensson P, et al. Quantitative and qualitative assessment of sensory changes induced by local anesthetics block of two different trigeminal nerve branches. Clin Oral Investig 2019;23(6):2637-2649. doi https://doi.org/10.1007/s00784-018-2695-4
- Bonica JJ, Loeser JD. Applied anatomy relevant to pain. In: Loeser JD, Butler SH, Chapman CR, Turk DC, editors. Bonica’s Management of Pain. 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 2001:196–221.
- Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain 2007;129(3):256-9. doi https://doi.org/10.1016/j.pain.2007.03.030
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between obesity and low back pain: a meta-analysis. Am J Epidemiol 2010;171:135–154. doi https://doi.org/10.1093/aje/kwp356
- Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123(3):231-43. doi https://doi.org/10.1016/j.pain.2006.01.041
- Blower PW. Neurologic patterns in unilateral sciatica. A prospective study of 100 new cases. Spine (Phila Pa 1976) 1981;6(2):175-9. doi https://doi.org/10.1097/00007632-198103000-00013
- Jonsson B, Stromqvist B. Symptoms and signs in degeneration of the lumbar spine. A prospective, consecutive study of 300 operated patients. J Bone Joint Surg Br 1993;75-B(3), 381–385. doi https://doi.org/10.1302/0301-620X.75B3.8496204
- Freynhagen R, Rolke R, Baron R, Tölle TR, Rutjes AK, Schu S, et al. Pseudoradicular and radicular low-back pain--a disease continuum rather than different entities? Answers from quantitative sensory testing. Pain 2008;135(1-2):65-74. doi https://doi.org/10.1016/j.pain.2007.05.004
- Scholz J, Mannion RJ, Hord DE, Griffin RS, Rawal B, Zheng H, et al. A novel tool for the assessment of pain: validation in low back pain. PLoS Med 2009;7;6(4):e1000047. doi https://doi.org/10.1371/journal.pmed.1000047
- Andrasinova T, Kalikova E, Kopacik R, Srotova I, Vlckova E, Dusek L, et al. Evaluation of the neuropathic component of chronic low back pain. Clin J Pain 2019;35(1):7-17. doi https://doi.org/10.1097/AJP.0000000000000653
- Tampin B, Slater H, Jacques A, Lind CRP. Association of quantitative sensory testing parameters with clinical outcome in patients with lumbar radiculopathy undergoing microdiscectomy. Eur J Pain 2020; 00:1–16. doi https://doi.org/10.1002/ejp.1586
- Raja SN, Ringkamp M, Guan Y, Campbell JN. John J. Bonica Award Lecture: Peripheral neuronal hyperexcitability: the “low-hanging” target for safe therapeutic strategies in neuropathic pain. Pain. 2020;161 Suppl 1(Suppl 1):S14-S26. doi: https://doi.org/10.1097/j.pain.0000000000001838
- Li J-Y, Xie W, Strong JA, Guo Q-L, Zhang J-M. Mechanical hypersensitivity, sympathetic sprouting, and glial activation are attenuated by local injection of corticosteroid near the lumbar ganglion in a rat model of neuropathic pain. Reg Anesth Pain Med. 2011;36(1):56-62. doi: https://doi.org/10.1097/AAP.0b013e318203087f
- Rolke R, Campbell AK, Magerl W, Treede RD. Deep pain thresholds in the distal limbs of healthy human subjects. Eur J Pain 2005;9(1):39‐48. doi https://doi.org/10.1016/j.ejpain.2004.04.001
- Hasvik E, Haugen AJ, Gjerstad J, Grøvle L. Assessing neuropathic pain in patients with low back-related leg pain: Comparing the painDETECT Questionnaire with the 2016 NeuPSIG grading system. Eur J Pain 2018;22(6):1160–9. doi https://doi.org/10.1002/ejp.1204
- Zhu GC, Böttger K, Slater H, et al. Concurrent validity of a low-cost and time-efficient clinical sensory test battery to evaluate somatosensory dysfunction. Eur J Pain. 2019;23(10):1826-1838. doi https://doi.org/10.1002/ejp.1456
- Moloney N, Hall T, Doody C. Divergent sensory phenotypes in nonspecific arm pain: comparisons with cervical radiculopathy. Arch Phys Med Rehabil 2015;96(2):269-275. doi https://doi.org/10.1016/j.apmr.2014.09.015
- Mironer YE, Somerville JJ. The current perception threshold evaluation in radiculopathy: Efficacy in diagnosis and assessment of the treatment results. Pain Digest 1998;8:37–8.
- Schiff E, Eisenberg E. Can quantitative sensory testing predict the outcome of epidural steroid injections in sciatica? A preliminary study. Anesth Analg 2003;97(3):828-32. doi https://doi.org/10.1213/01.ane.0000078583.47735.69
- Bialosky JE, Bishop MD, Robinson ME, Zeppieri G Jr, George SZ. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther. 2009;89(12):1292-1303. doi https://doi.org/10.2522/ptj.20090058
- Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain. 2019;160(9):1920-1932. doi https://doi.org/ 10.1097/j.pain.0000000000001590