Acta medica Lituanica ISSN 1392-0138 eISSN 2029-4174
2021. Online ahead of print DOI: https://doi.org/10.15388/Amed.2021.28.1.21
First Detection of Human Coronavirus HKU1 in Greece, in an Immunocompromised Patient With Severe Lower Respiratory Tract Infection
Vasiliki Epameinondas Georgakopoulou*
Pulmonology Department, Laiko General Hospital, Athens, Greece
Georgios Petsinis
1st Pulmonology Department Sismanogleio Hospital, Athens, Greece
Konstantinos Mantzouranis
1st Pulmonology Department Sismanogleio Hospital, Athens, Greece
Christos Damaskos
Second Department of Propedeutic Surgery, Laiko General Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
N.S. Christeas Laboratory of Experimental Surgery and Surgical Research, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Despoina Melemeni
2 1st Pulmonology Department Sismanogleio Hospital, Athens, Greece
Aikaterini Gkoufa
First Department of Internal Medicine, Laiko General Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Serafeim Chlapoutakis
Department of Thoracic Surgery, Agios Savvas Hospital, Athens, Greece
Nikolaos Garmpis
Second Department of Propedeutic Surgery, Laiko General Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
N.S. Christeas Laboratory of Experimental Surgery and Surgical Research, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Pagona Sklapani
Department of Cytology, Mitera Hospital, Athens, Greece
Nikolaos Trakas
Department of Biochemistry, Sismanogleio Hospital, Athens, Greece
Xanthi Tsiafaki
1st Pulmonology Department Sismanogleio Hospital, Athens, Greece
Abstract. Human coronavirus HKU1 (HCoV-HKU1) is a RNA virus which gets in the human cells by binding to the receptor of N-acetyl-9-O-acetylneuraminic acid. Human Coronaviruses (HCoVs), including HCoV-HKU1, are globally found. HCoV-HKU1 is responsible for upper and lower respiratory tract infections, usually with mild symptoms. In severe cases, HCoV-HKU1 can cause life-threatening respiratory illness especially in vulnerable hosts such as elderly, children and immunocompromised patients. In Greece, Respiratory Syncytial Virus (RSV) and influenza are the most common viruses causing respiratory tract infections. Traditionally, HCoVs are responsible for less than 3% of respiratory infections in Greek population. HCoVs 229E and OC43 have been shown to circulate in Greece. We report the first case of lung infection in an immunocompromised woman due to HCoV-HKU1, that has never been before detected in Greece. HCoV-HKU1 is related to severe disease even in healthy individuals and must be considered in the differential diagnosis of severe respiratory infections.
Keywords: HCoV-HKU1, Human Coronaviruses, Pneumonia, Immunosuppression
Pirmasis žmogaus koronaviruso HKU1 atvejis Graikijoje, nustatytas pacientui, turinčiam imuninės sistemos sutrikimą ir sergančiam sunkia apatinių kvėpavimo takų infekcija
Santrauka. Žmogaus koronavirusas HKU1 (HCoV-HKU1) yra RNR virusas, jis patenka į žmogaus organizmo ląsteles, jungdamasis prie N-9-O-acetilneuramino rūgšties receptoriaus.
Žmogaus koronavirusai (hCoVs), įskaitant HCoV-HKU1, yra aptinkami visame pasaulyje. HCoV-HKU1 sukelia viršutinių ir apatinių kvėpavimo takų infekcijas, tačiau paprastai simptomai yra nežymūs. Sudėtingu atveju HCoV-HKU1gali sukelti gyvybei pavojingą kvėpavimo takų ligą, ypač asmenims, priklausantiems pažeidžiamoms asmenų grupėms, pavyzdžiui, vyresnio amžiaus žmonėms, vaikams ir pacientams, kurių imuninė sistema yra sutrikusi. Graikijoje respiracinis sincitinis virusas (RSV) ir gripo virusas yra dažniausi virusai, sukeliantys kvėpavimo takų infekcijas. Tradiciškai hCoVs sukelia mažiau negu 3 % kvėpavimo takų infekcijų Graikijos populiacijoje. Paaiškėjo, kad virusai HCoVs 229E ir OC43 vyrauja Graikijoje. Straipsnyje pristatome pirmąjį plaučių infekcijos, kurią sukėlė HCoV-HKU1 moteriai, turinčiai sutrikusį imunitetą, atvejį, tokio niekada anksčiau nebuvo nustatyta Graikijoje. HCoV-HKU1 sukelia sunkią ligą net ir sveikiems asmenims ir į tai būtina atsižvelgti diferencinėje sunkių kvėpavimo takų infekcijų diagnostikoje.
Reikšminiai žodžiai: HCoV-HKU1, žmogaus koronavirusai, pneumonija, imunosupresija
_______
* Corresponding author: Vasiliki Epaneinondas Georgakopoulou, Pulmonology Department, Laiko General Hospital, 17 Agiou Thoma Street, 11527, Athens, Greece. Tel number: +00306938103639. E-mail: vaso_georgakopoulou@hotmail.com
Received: 15/03/2021. Revised: 30/04/2021. Accepted: 06/05/2021
Copyright © 2021 Vasiliki Epameinondas Georgakopoulou, Georgios Petsinis, Konstantinos Mantzouranis, Christos Damaskos, Despoina Melemeni, Aikaterini Gkoufa, Serafeim Chlapoutakis, Nikolaos Garmpis, Pagona Sklapani, Nikolaos Trakas, Xanthi Tsiafaki. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Human coronavirus HKU1 (HCoV-HKU1) is a coronavirus species in humans. It is an enveloped, RNA virus which gets in the human cells by binding to the receptor of N-acetyl-9-O-acetylneuraminic acid [1]. It has the Hemagglutinine estarase (HE) gene, which differentiates it as a member of the genus Betacoronavirus and subgenus Embecovirus [2].
HCoV-HKU1 was first described in a 71-year-old man who had returned to Hong Kong from Shenzhen, China in 2004 and developed bilateral pneumonia and acute respiratory distress syndrome [3]. Human Coronaviruses (HCoVs), including HCoV-HKU1, are globally found in the humans and are responsible for approximately one-third of human common cold infections. In severe cases, they can cause life-threatening pneumonia and bronchiolitis especially in vulnerable hosts such as elderly, children and immunocompromised patients. In addition to respiratory diseases, they also cause gastrointestinal and neurological illnesses [1,4].
In Greece, according to large epidemiological studies, Respiratory Syncytial Virus (RSV) and influenza are the most common viruses responsible for respiratory tract infections. Traditionally, HCoVs are responsible for less than 3% of respiratory infections in Greek population [5,6]. We report the first case of lung infection in an immunocompromised woman due to HCoV-HKU1, that has never been before detected in Greece.
Case Report
A 67-year-old woman, with a history of arterial hypertension, diabetes mellitus type II, thyroidectomy, osteoporosis, hyperlipidemia and rheumatoid arthritis being treated with methotrexate and corticosteroids, presented to our Pulmonology Department in August 2019 with progressive dyspnea at rest and fatigue over the last two days.
Clinical examination revealed an afebrile patient with crackles on auscultation at all lung fields. Blood pressure was 100/80 mmHg, heart rate was 120 beats per minute, oxygen saturation was 79% on room air and body temperature 36.5 °C on admission. Electrocardiography showed sinus tachycardia. Arterial blood gas analysis revealed pO2 40mmHg, pCO2 35mmHg, pH 7.48 and HCO3¯ 29.2 mmol/L on room air. Chest X-ray showed patchy diffuse infiltrates in both lungs mostly in the left lower lobe (Figure 1A).
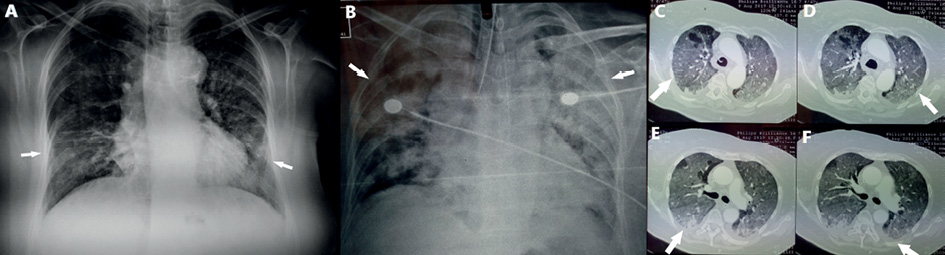
Figure 1. A: Chest X-ray shows patchy diffuse infiltrates in both lungs mostly in the left lower lobe on admission. B: Chest X-ray shows diffuse lung infiltrates after two days of hospitalization.
C, D: Chest Computed Tomography reveals diffuse ground glass opacities. E, F: Chest Computed Tomography reveals pleural effusion in both lungs.
Complete blood count was normal. The blood biochemistry parameters and thyroid-stimulating hormone (TSH) were normal, with the exception of an elevated serum lactate dehydrogenase (LDH) 311 U/L (normal <225 U/L), serum glycose 263 mg/dl (normal 60-100 mg/dL) and C-reactive protein (CRP) 122.3 mg/L (normal <6 mg/L). Urinalysis was normal.
The reversed transcription polymerase chain reaction (RΤ-PCR) test for Influenza A and B in nasopharyngeal and oropharyngeal samples were performed and were negative. Urinary antigen tests for Legionella pneumophila and Streptococcus pneumoniae, serological testing for Mycoplasma pneumonia and blood cultures were negative.
The patient received oxygen therapy with Venturi mask delivering 60% oxygen and intravenous antibiotic therapy with ceftriaxone, azithromycin and trimethoprim-sulfomethoxazole empirically. After the first two days of hospitalization the patient presented with respiratory deterioration and diffuse lung infiltrates (Figure 1B), was intubated and admitted to intensive care unit.
Computed tomography (CT) of the chest was performed. Chest CT revealed diffuse ground glass opacities in all lung fields (Figure 1C, 1D) and bilateral pleural effusion (Figure 1E, 1F). The patient underwent echocardiography with normal ejection fraction and valve function. Bronchoscopy was performed and bronchial washings and bronchoalveolar lavage (BAL) were obtained from lingula and right middle lobe. Cytological and microbiological examination of bronchial washings and BAL was negative. Direct immunofluorescence and PCR for Pneumocystis jirovecii in BAL were negative and administration of trimethoprim-sulfomethoxazole was discontinued. Respiratory virus panel test in BA for detection of human respiratory viruses by use of multiplex RT-PCR was performed and revealed HCoV-HKU1.
The patient after five days of intubation presented with improvement, was extubated and received oxygen therapy with Venturi mask. The patient had gradually completely recovered without any specific therapy. She denied recent travel or contact with a person that had recently returned from a travel abroad.
Discussion
HCoV-HKU1 is related to both upper and lower respiratory tract infections. The most common method for diagnosing HCoV-HKU1 infection is RT-PCR or real-time RT-PCR using RNA extracted from respiratory tract samples. HCoV-HKU1 infections have the highest incidence in winter, however they occur also in spring and summer [7]. HCoV-HKU1-associated pneumonia is a monophasic disease, and most patients have mild symptoms that were localized to the respiratory tract. Most patients with HCoV-HKU1-associated pneumonia are >65 years old. There is currently no treatment recommended for HCoV-HKU1-associated pneumonia except for supportive care as needed [8].
HCoV-HKU1 has been associated with acute respiratory illness in patients with underlying disease such as immunosuppression. Fatal pneumonia due to HCoV-HKU1 has been described in patients with diabetes mellitus, malignancy and cardiovascular disease [8]. HCoV-HKU1 has been detected as causative agent of severe respiratory infection in older adults with Chronic Obstructive Pulmonary Disease (COPD) [9]. Lower respiratory tract infections due to HCoV-HKU1 in patients with hematopoietic stem cell transplantation or hematological malignancies are associated with high rates of oxygen use and mortality [10]. In addition, HCoV-HKU1 has been reported to cause diffuse pneumonitis in a 65-year-old man diagnosed with stage IV melanoma who developed pulmonary and brain metastases and was treated with combined nivolumab and ipilimumab immunotherapy [11].
HCoV-HKU1 is considered to have global distribution with a median incidence of 0.9 (0–4.4) % [7, 12]. It has been described to be responsible for 2.1% of respiratory infections due to HCoVs in Kenya during 2009-2012 [13], 16.6% of respiratory infections due to HCoVs in Yamagata, Japan during 2010-2019 [14], 0.13% of respiratory infections due to HCoVs in Hong Kong during 2008-2014 [15], 7.82% of respiratory infections due to HCoVs in Guangzhou, China during 2010–2015 [16], 1.6% of all respiratory infections in Cleveland, Ohio in 2016 [17], 1.1% of all respiratory infections in Kuala Lumpur, Malaysia [18], 0.6% of respiratory infections due to HCoVs in the United States during 2014–2017 [19] and 0.32% of respiratory infections due to HCoVs in Thailand during 2012–2013 [20].
HCoV-HKU1, HCoV 229E, NL63 and OC43 are known as nonsevere acute respiratory syndrome (SARS)-like CoVs while the other three HCoVs, SARS-CoV-1, MERS-CoV and SARS-CoV-2 are thought to be highly pathogenic, causing lethal human disease [12]. However, HCoV-HKU1 has been associated with severe and fatal respiratory infection in patients without underlying diseases, between 2013 and 2017, in Brazil [21] and with severe acute respiratory illness
among patients hospitalized in South Africa during 2012-2013 [22].
In addition, HCoV-HKU1 has been detected in patients with respiratory infections in European countries. HCoV-HKU1 was detected in nasal samples and stool samples from patients hospitalized for respiratory infection in February and March 2005 at the University Hospital of Caen, France [23]. Moreover, HCoV-HKU1 was the cause of acute respiratory tract infections in patients hospitalized in Pavia, Italy during the period January-May 2006 [24].
In Greece, data about epidemiological and clinical aspects of HCoVs are limited.
HCoVs 229E and OC43 have been shown to circulate in Greek population [5]. To our knowledge, HCoV-HKU1 has never been before detected in Greece. A further investigation about clinical and molecular epidemiology of HCoVs is needed in Greece.
Conclusions
This is the first case of isolation of HCoV-HKU1 in a patient with pneumonia in Greece. HCoVs are a common cause of respiratory infections, responsible for considerable morbidity and hospitalization of all age groups, especially in immunocompromised patients. Moreover, it is of great importance to include Coronaviruses in diagnostic panels used by official surveillance systems because along with their pandemic potential, endemic HCoVs, including HCoV-HKU1, are related to severe disease even in healthy individuals and must be considered in the differential diagnosis of severe respiratory infections.
Conflicts of Interest
None
Acknowledgements
None
Financial Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Written Consent
Written consent has been obtained from the patient referred in this case report
References
- Lim YX, Ng YL, Tam JP, Liu DX. Human Coronaviruses: A Review of Virus-Host Interactions. Diseases. 2016;4(3):26. doi: https://doi.org/10.3390/diseases4030026.
- Woo PC, Huang Y, Lau SK, Yuen KY. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8):1804-20. doi: https://doi.org/10.3390/v2081803.
- Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005 Jan;79(2):884-95. doi: https://doi.org/10.1128/JVI.79.2.884-895.2005.
- Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and Sources of Endemic Human Coronaviruses. Adv Virus Res. 2018;100:163-188. doi: https://doi.org/10.1016/bs.aivir.2018.01.001.
- Pogka V, Kossivakis A, Kalliaropoulos A, et al. Respiratory viruses involved in influenza-like illness in a Greek pediatric population during the winter period of the years 2005-2008. J Med Virol. 2011;83(10):1841-8. doi: https://doi.org/10.1002/jmv.22173.
- Antalis E, Kottaridi C, Kossyvakis A, et al. Clinical and Molecular Epidemiology of Respiratory Viruses in a Tertiary Care Center During the 2009–2014 Consecutive Winter Seasons Open Forum Infect Dis. 2015;2(Suppl 1):545. doi: https://doi.org/10.1093/ofid/ofv133.420.
- Woo PC, Lau SK, Yip CC, et al. More and More Coronaviruses: Human Coronavirus HKU1. Viruses. 2009;1(1):57-71. doi: https://doi.org/10.3390/v1010057
- Woo PC, Lau SK, Tsoi HW, et al. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005 Dec 1;192(11):1898-907. doi: https://doi.org/10.1086/497151.
- Gorse GJ, O’Connor TZ, Hall SL,et al. Human coronavirus and acute respiratory illness in older adults with chronic obstructive pulmonary disease. J Infect Dis. 2009;199(6):847-57. doi: https://doi.org/10.1086/597122.
- Ogimi C, Kim YJ, Martin ET, et al. What’s New With the Old Coronaviruses? J Pediatric Infect Dis Soc. 2020;9(2):210-217. doi: https://doi.org/10.1093/jpids/piaa037.
- Serzan MT, Kumar PN, Atkins MB. Diffuse pneumonitis from coronavirus HKU1 on checkpoint inhibitor therapy. J Immunother Cancer. 2020;8(1):e000898. doi: https://doi.org/10.1136/jitc-2020-000898.
- Raoult D, Zumla A, Locatelli F, et al. Ippolito G, Kroemer G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4(4):66-75. doi: https://doi.org/10.15698/cst2020.04.216.
- Sipulwa LA, Ongus JR, Coldren RL, et al. Molecular characterization of human coronaviruses and their circulation dynamics in Kenya. Virol J. 2016;13:18. doi: https://doi.org/10.1186/s12985-016-0474-x.
- Komabayashi K, Seto J, Matoba Y, et al.Seasonality of Human Coronavirus OC43, NL63, HKU1, and 229E Infection in Yamagata, Japan, 2010-2019. Jpn J Infect Dis. 2020;73(5):394-397. doi: https://doi.org/10.7883/yoken.JJID.2020.525.
- Yip CC, Lam CS, Luk HK, et al. A six-year descriptive epidemiological study of human coronavirus infections in hospitalized patients in Hong Kong. Virol Sin. 2016;31(1):41-8. doi: https://doi.org/10.1007/s12250-016-3714-8.
- Zhang SF, Tuo JL, Huang XB, et al. PLoS One. 2018;13(1):e0191789. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010-2015 in Guangzhou. doi: https://doi.org/10.1371/journal.pone.0191789.
- Kanwar A, Selvaraju S, Esper F. Human Coronavirus-HKU1 Infection Among Adults in Cleveland, Ohio. Open Forum Infect Dis. 2017;4(2):ofx052. doi: https://doi.org/10.1093/ofid/ofx052.
- Al-Khannaq MN, Ng KT, Oong XY, et al. Molecular epidemiology and evolutionary histories of human coronavirus OC43 and HKU1 among patients with upper respiratory tract infections in Kuala Lumpur, Malaysia. Virol J. 2016;13:33. doi: https://doi.org/10.1186/s12985-016-0488-4.
- Killerby ME, Biggs HM, Haynes A, et al. Human coronavirus circulation in the United States 2014-2017. J Clin Virol. 2018;101:52-56. doi: https://doi.org/10.1016/j.jcv.2018.01.019.
- Soonnarong R, Thongpan I, Payungporn S, et al. Molecular epidemiology and characterization of human coronavirus in Thailand, 2012–2013. Springerplus. 2016;5(1):1420. doi: https://doi.org/10.1186/s40064-016-3101-9.
- Veiga ABGD, Martins LG, Riediger I, et al.More than just a common cold: Endemic coronaviruses OC43, HKU1, NL63, and 229E associated with severe acute respiratory infection and fatality cases among healthy adults. J Med Virol. 2020. doi: https://doi.org/10.1002/jmv.26362.
- Subramoney K, Hellferscee O, Pretorius M, et al. Human bocavirus, coronavirus, and polyomavirus detected among patients hospitalised with severe acute respiratory illness in South Africa, 2012 to 2013. Health Sci Rep. 2018;1(8):e59. doi: https://doi.org/10.1002/hsr2.59.
- Vabret A, Dina J, Gouarin S, Petitjean J, et al. Detection of the New Human Coronavirus HKU1: A Report of 6 Cases. Clin Infect Dis. 2006;42(5):634-9. doi: https://doi.org/10.1086/500136.
- Gerna G, Percivalle E, Sarasini A, et al. Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J Clin Virol. 2007;38(3):244-250. doi: https://doi.org/10.1016/j.jcv.2006.12.008.