Acta medica Lituanica ISSN 1392-0138 eISSN 2029-4174
2022. Online ahead of print DOI: https://doi.org/10.15388/Amed.2022.29.2.12
Incidentally Diagnosed Extraluminal Leiomyosarcoma of Infrarenal Inferior Vena Cava: A Case Report and Literature Review from a Radiologist’s Perspective
Ebinesh A*
Department of Radiodiagnosis, Maulana Azad Medical College and associated hospitals, Jawahar Lal Nehru Marg, New Delhi, India
Aanchal Ashta
Department of Radiology, Maulana Azad Medical College and associated hospitals, Jawahar Lal Nehru Marg, New Delhi, India
Satyam
Department of Radiodiagnosis, Maulana Azad Medical College and associated hospitals, Jawahar Lal Nehru Marg, New Delhi, India
Gaurav Shanker Pradhan
Department of Radiodiagnosis, Maulana Azad Medical College and associated hospitals, Jawahar Lal Nehru Marg, New Delhi, India
Rohin Sharma
Department of Radiodiagnosis, Maulana Azad Medical College and associated hospitals, Jawahar Lal Nehru Marg, New Delhi, India
Prince Das
Department of Radiology, Maulana Azad Medical College and associated hospitals, Jawahar Lal Nehru Marg, New Delhi-110002, India
Abstract. Background: Vascular leiomyosarcoma is a rare but most common vascular tumor of the inferior vena cava.
Case presentation: We present the case of an incidentally diagnosed extraluminal leiomyosarcoma of the inferior vena cava in a 62 year old patient who presented with abdominal pain following blunt trauma. Ultrasonography showed a lobulated hypoechoic lesion in the upper abdomen. Computed tomography (CT) and magnetic resonance imaging (MRI) showed a circumscribed lobulated near homogeneously enhancing retroperitoneal lesion in anterior relation to the infrarenal inferior vena cava, right paramedian in location with imperceptible vena caval lumen at the site of maximum contact. In positron emission tomography (PET) CT the lesion showed mild fluorodeoxyglucose (FDG) uptake with no distant metastases. CT guided biopsy with immunohistochemical analysis showed leiomyosarcoma. Patient underwent surgical resection with inferior vena cava reconstruction.
Conclusions: Leiomyosarcoma of the inferior vena cava is a rare tumor of vascular origin. Imaging plays an imperative role in the diagnosis and preoperative evaluation. This article also provides a comprehensive literature review of the radiological features of inferior vena caval leiomyosarcoma that would aid in optimal preoperative characterization and evaluation.
Keywords: Leiomyosarcoma, inferior vena cava, retroperitoneal tumor, imaging features.
Atsitiktinai diagnozuota neluminalinės infrarenalinės apatinės tuščiosios venos lejomiosarkoma: atvejo aprašymas ir literatūros apžvalga radiologo požiūriu
Santrauka. Įvadas: Kraujagyslių lejomiosarkoma yra retas, tačiau dažniausiai pasitaikantis apatinės tuščiosios venos navikas.
Atvejo aprašymas: 62 metų pacientui, kuris kreipėsi į gydymo įstaigą dėl pilvo srities skausmo po neaštriu daiktu sukeltos traumos, buvo atsitiktinai nustatyta neluminalinės infrarenalinės apatinės tuščiosios venos lejomiosarkoma.
Ultragarso tyrimas atskleidė skiltinį hipoechogenišką pažeidimą viršutinėje pilvo srityje. Kompiuterinė tomografija (KT) ir magnetinio rezonanso tomografija (MRT) parodė, kad priešakinėje infrarenalinėje apatinėje tuščiojoje venoje yra apibrėžtas skiltinis, beveik homogeniškai intensyvėjantis retroperitoninis pažeidimas, kuris didžiausio sąlyčio vietoje su tuščiosios venos spindžiu į dešinę nuo centro yra beveik nematomas. Pozitronų emisijos tomografijos (PET) metu pažeidimas parodė nedidelį fluorodeoksigliukozės (FDG) įsisavinimą be jokių tolimų metastazių. Atlikus kompiuterine tomografija paremta biopsiją su imunohistochemine analize, nustatyta lejomiosarkoma. Pacientui buvo atlikta chirurginė rezekcija ir apatinės tuščiosios venos rekonstrukcija.
Išvados: Apatinės tuščiosios venos lejomiosarkoma yra retas kraujagyslių sistemos auglys. Diagnozei ir vertinimui iki operacijos būtina peržiūrėti vaizdinę medžiagą. Šiame straipsnyje taip pat pateikiama išsami literatūros apžvalga apie radiologines apatinės tuščiosios venos lejomiosarkomos savybes, kurios pagerintų optimalų priešoperacinį įvertinimą.
Raktažodžiai: Lejomiosarkoma, apatinė tuščioji vena, retroperitoninis navikas, vaizdavimo ypatybės
__________
* Corresponding author: Ebinesh A. Department of Radiodiagnosis, Maulana Azad Medical College and associated hospitals, Jawahar Lal Nehru Marg, New Delhi-110002, India. E-mail: ebineshjezreel@gmail.com
Received: 17/08/2022. Revised: 04/10/2022. Accepted: 10/10/2022
Copyright © 2022 Ebinesh A, Aanchal Ashta, Satyam, Gaurav Shanker Pradhan, Rohin Sharma, Prince Das. Published by Vilnius University Press.This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Leiomyosarcoma (LMS) of inferior vena cava is a rare malignant tumor originating from the smooth muscle layer of the tunica media. It was first reported by Paerl et al [1] in 1871. Leiomyosarcomas of vascular origin are rare with an incidence of less than 2% [2]. Occurrence of leiomyosarcoma of the inferior vena cava is exceedingly rare, accounting for 0.5 % of all adult sarcomas [3]. These tumors are asymptomatic or present with nonspecific symptoms such as anorexia, vomiting, loss of weight and abdominal pain and shows female preponderance. At rare instances, the presentation as Budd–Chiari syndrome has also been reported earlier [4,5]. Definitive treatment is surgical excision and prognosis is generally poor [6].
In this report, we present the case of an extraluminal leiomyosarcoma incidentally diagnosed in a 62 year old male patient who presented with abdominal pain following blunt abdominal trauma. Owing to the paucity of literature on radiological features of these tumors, we also present a comprehensive review of the crucial radiological aspects for evaluation.
Case report
A 62 year old male presented with complaints of pain in abdomen following blunt trauma to abdomen. On examination, he was afebrile with stable vitals. Palpation of the abdomen revealed tenderness in the upper abdomen and a firm palpable mass along the midline of upper abdomen.
Routine ultrasonographic examination revealed a circumscribed hypoechoic lesion in the upper abdomen measuring 6.6 × 6.3 × 5.9 cm (CC × AP × Tr). For further evaluation, patient underwent computed tomography which revealed a circumscribed retroperitoneal soft tissue lesion in right side of midline, measuring 7.3 × 7.5 × 8.1cm (CC × AP × Tr). The lesion showed inhomogeneous contrast uptake on arterial phase and homogeneous enhancement on portal venous and delayed phases with few nonenhancing areas within. It was abutting the inferior vena cava posteriorly with imperceptible lumen at the site of maximum contact. However, proximal and distal segments of the inferior vena cava showed normal contrast opacification. Medially, the lesion was in relation to the abdominal aorta. Second and third part of duodenum and head of pancreas were displaced anterosuperiorly. Magnetic resonance imaging (MRI) showed a homogeneously enhancing circumscribed lesion in the retroperitoneum on right side of midline appearing isointense on T1W, hyperintense on T2W showing restricted diffusion. Few cystic areas were seen within the lesion. On prone imaging, the lumen of inferior vena cava was still imperceptible at the site of maximum contact. No obvious intraluminal extension was seen. Whole body positron emission tomography CT revealed mild FDG uptake with a maximum standard uptake value (SUVmax) of 4.1. No distant metastases were found.
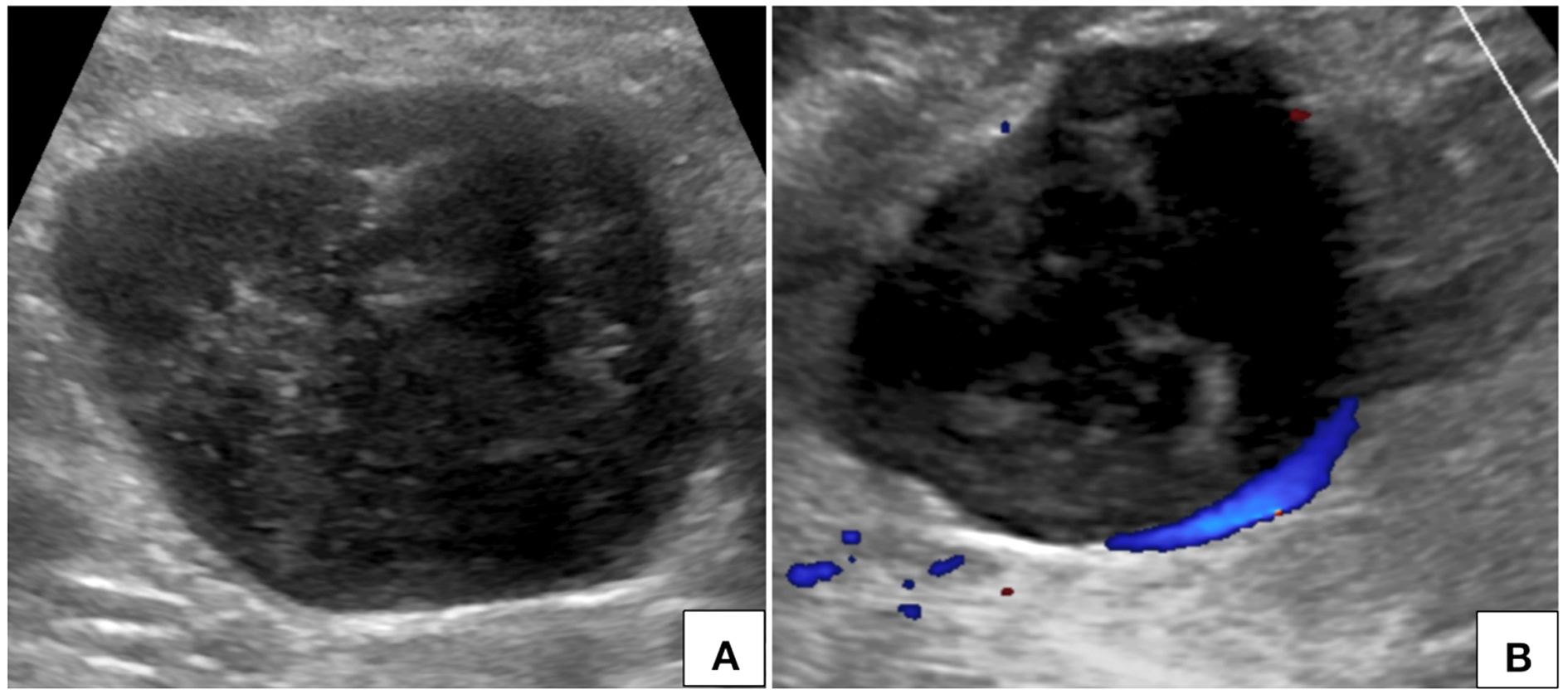
Figure 1. Ultrasonography of abdomen (A) with color Doppler flow imaging (B) shows a heterogeneously hypoechoic circumscribed lesion in the upper abdomen in right paramedian location with no significant internal vascularity.
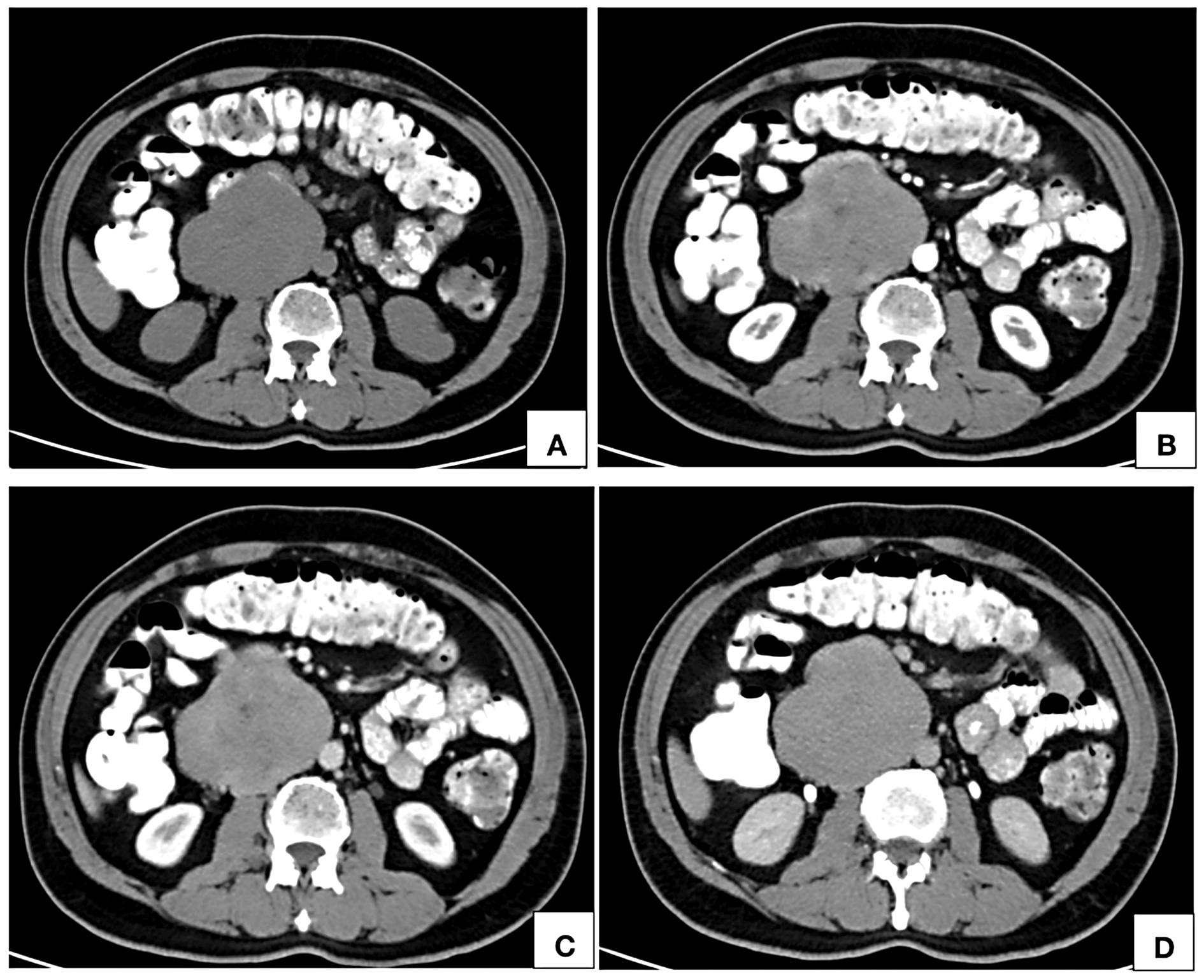
Figure 2. Computed tomographic axial images of noncontrast (A), arterial phase (B), portal venous phase (C) and delayed phase (D) show an isodense (A) retroperitoneal lesion right paramedian in location, in close contact with the abdominal aorta showing inhomogeneous areas of contrast uptake on arterial phase (B) with homogeneous enhancement on portal venous (C) and delayed phases (D). The lumen of inferior vena cava at the site of maximum contact is imperceptible.
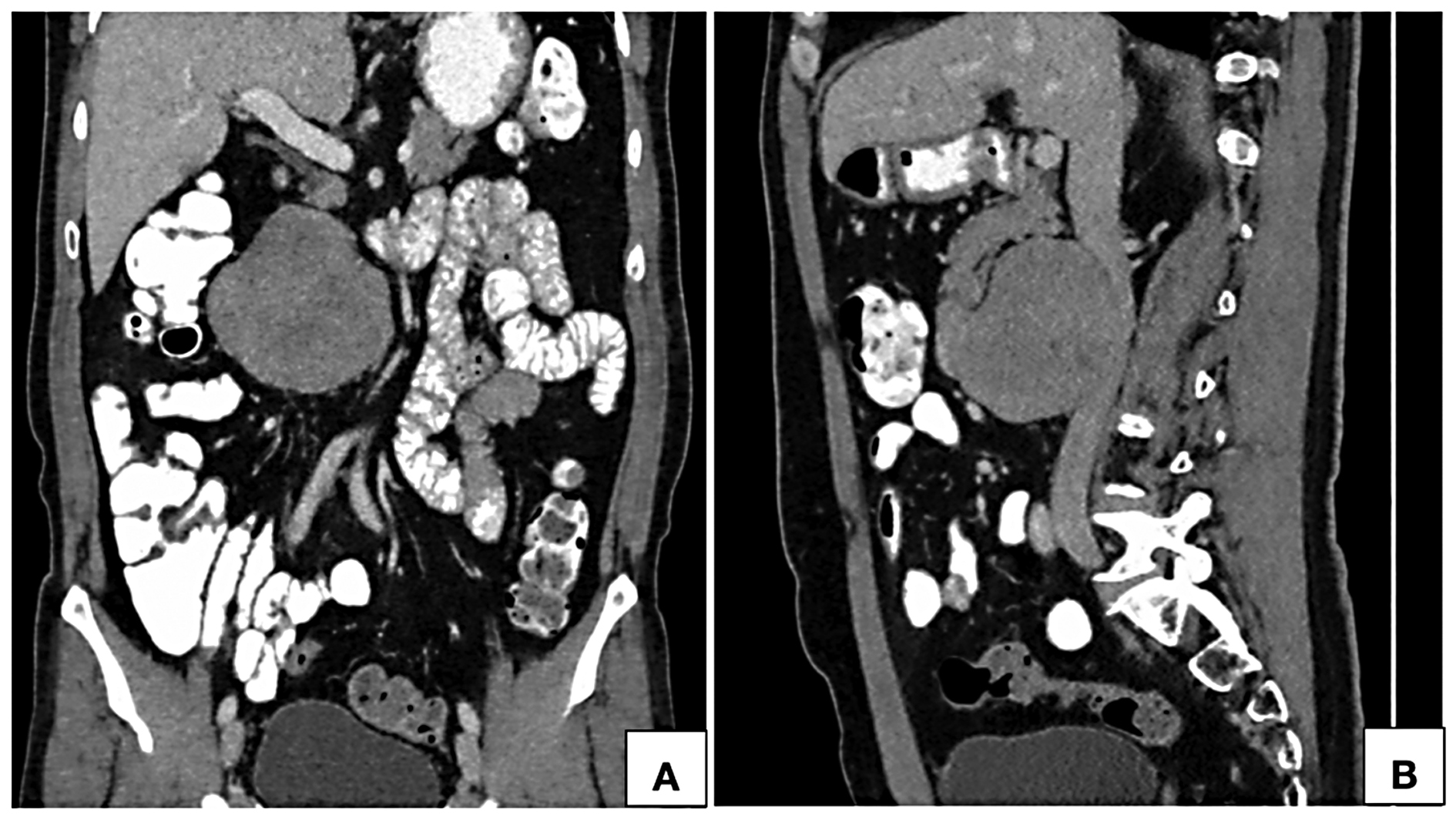
Figure 3. Computed tomographic coronal (A) and sagittal (B) reformatted images in portal venous phase show a near homogeneously enhancing retroperitoneal lesion in anterior relation to the infrarenal inferior vena cava towards the right of the midline. Proximal and distal segments of the inferior vena cava show normal contrast opacification with no intraluminal component.
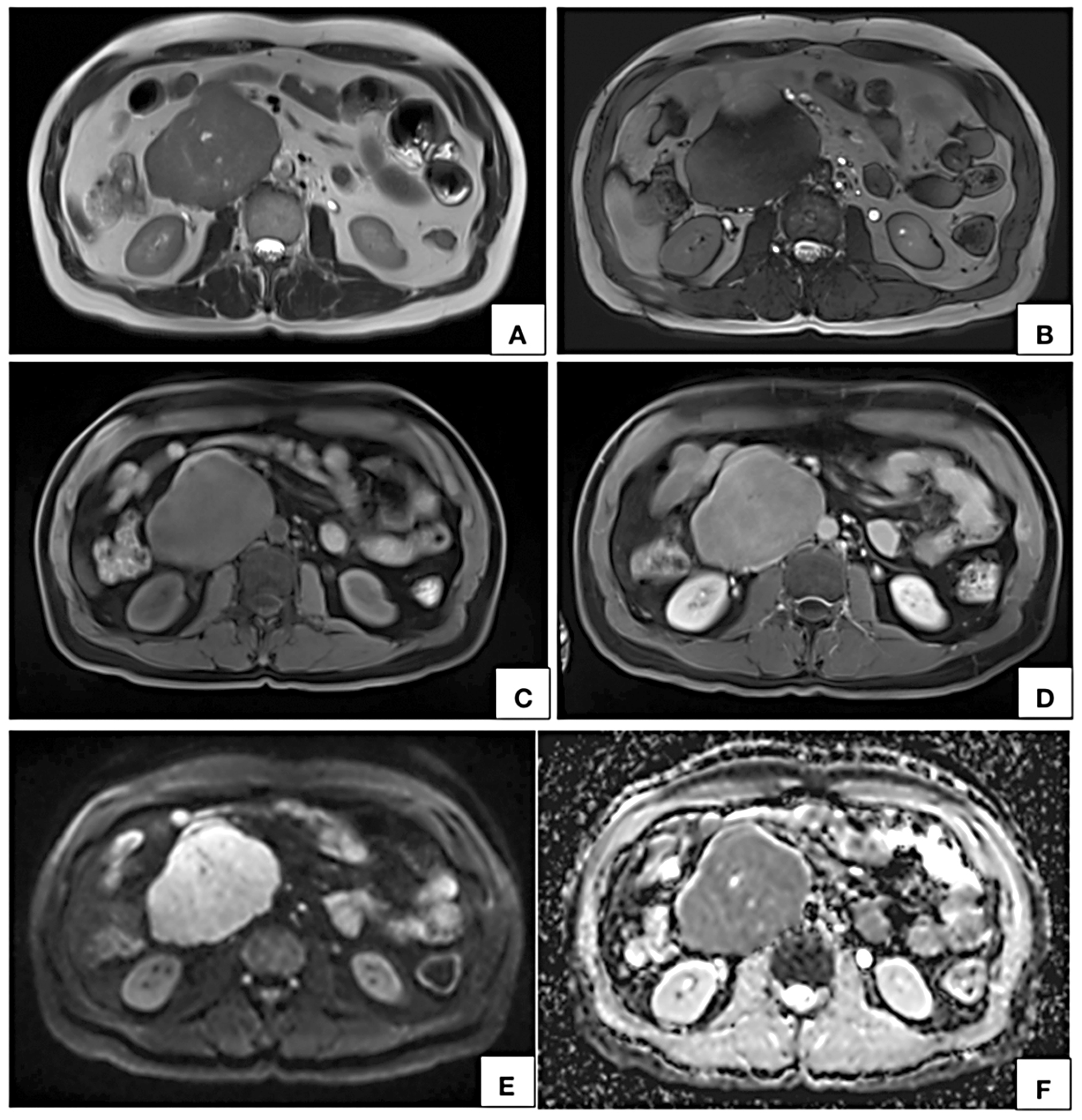
Figure 4. MRI axial T2W (A), T2W TRUFI (B), precontrast (C) and post contrast (D) T1W fat saturated VIBE images show a circumscribed lobulated retroperitoneal lesion on the right side of midline appearing hyperintense on T2W (A) and hypointense on T1W (C) showing near homogeneous contrast enhancement (D). In axial DWI (E) image and corresponding ADC map (F), the lesion shows restricted diffusion. Few T2W hyperintense nonenhancing cystic areas are seen within the lesion.
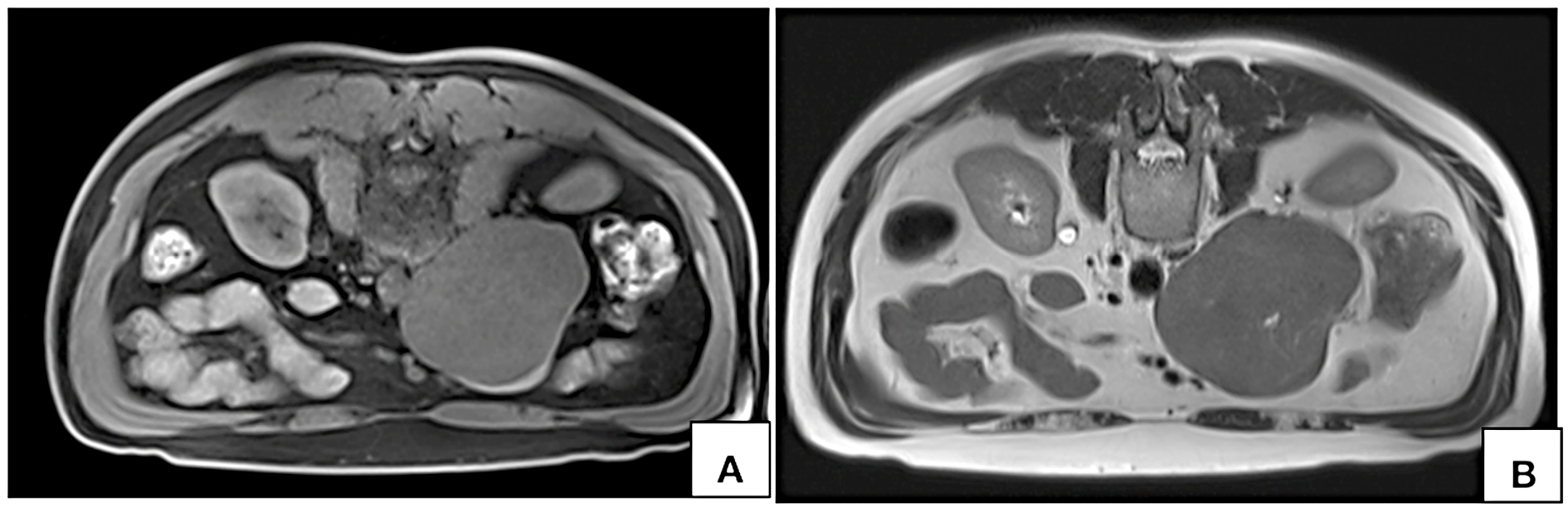
Figure 5. MRI axial T1W fat saturated VIBE (A) and T2W (B) images acquired in prone position show persistent nonvisualization of the lumen on inferior vena cava at the site of maximum contact.
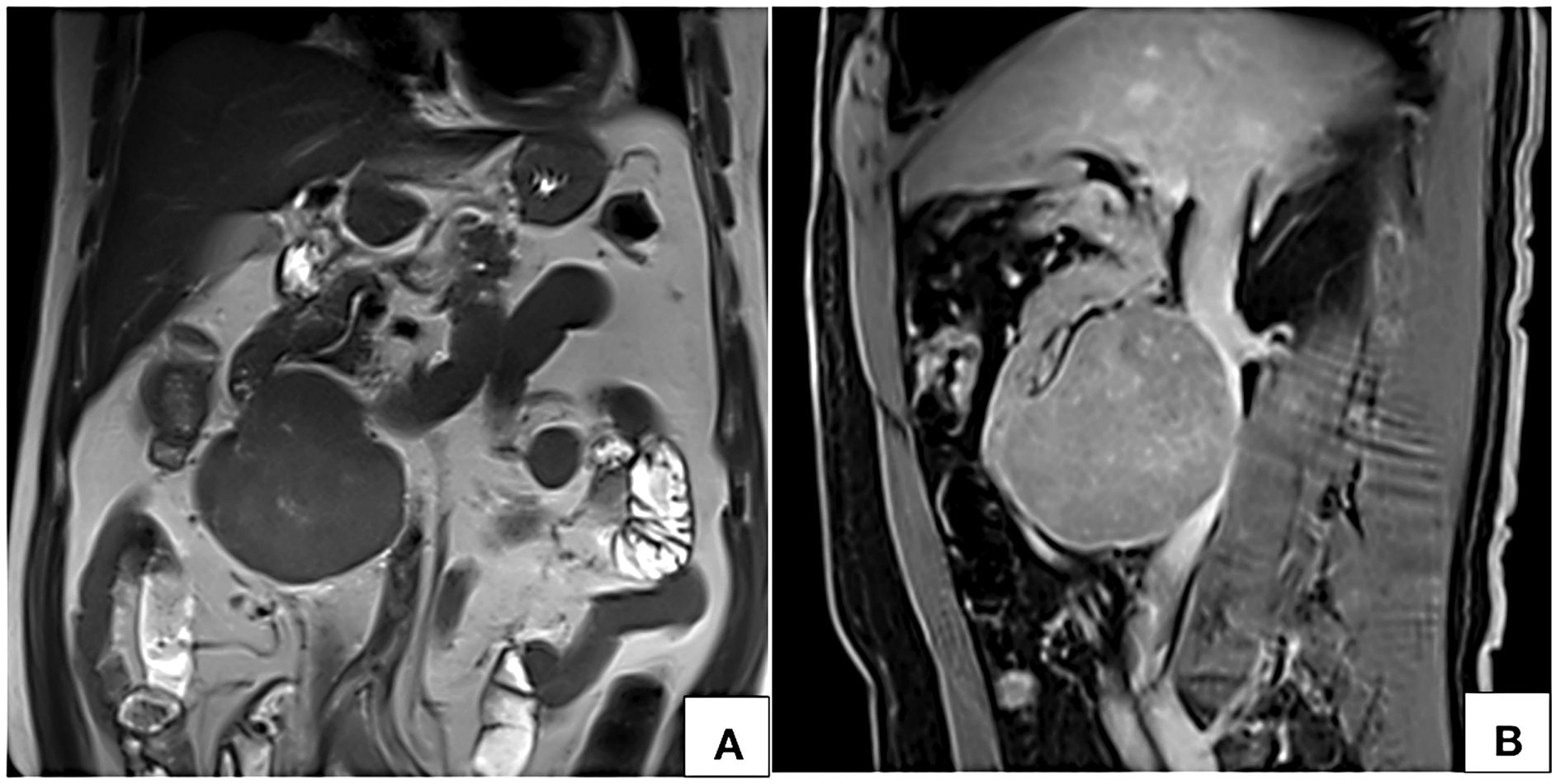
Figure 6. MRI coronal T2W (A) and sagittal post contrast T1W fat saturated VIBE (B) images show a circumscribed near homogeneously enhancing lesion along the anterior wall of infrarenal inferior vena cava with no intraluminal filling defect.
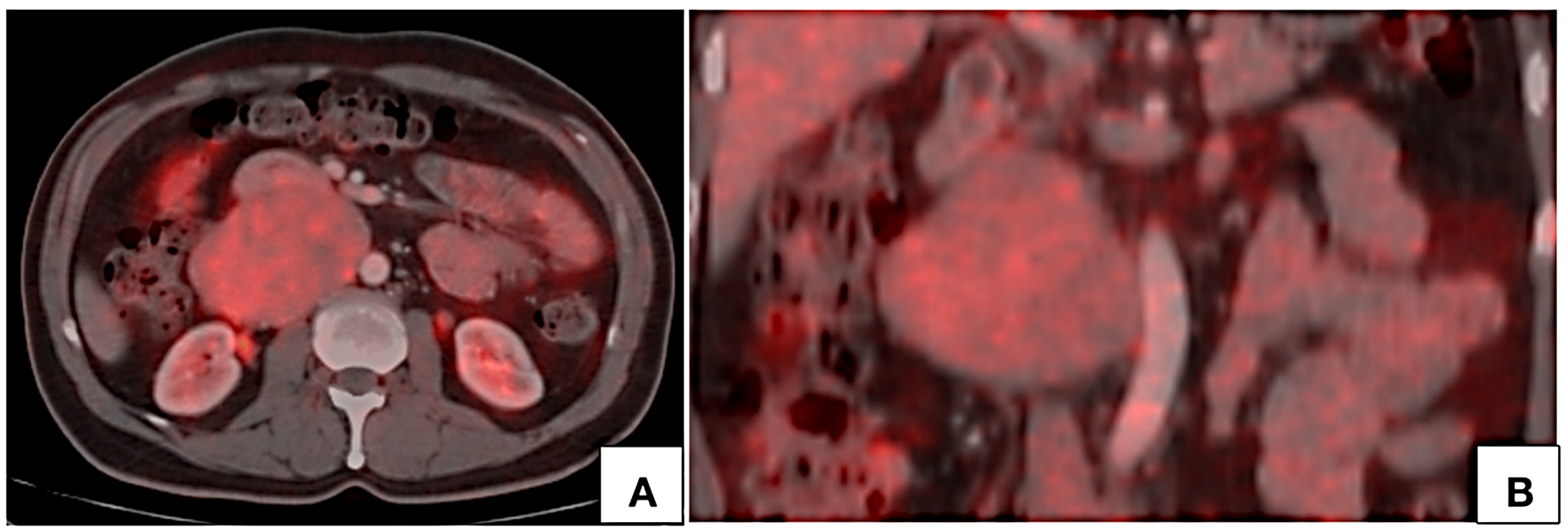
Figure 7. PET CT axial (A) and coronal (B) images show mild FDG uptake by the lesion with no hypermetabolic lymph nodes.
Based on the radiological findings, differential diagnoses included low grade retroperitoneal lymphoma and primary retroperitoneal sarcoma. Histopathological sampling was done under computed tomographic guidance. Histopathological examination showed malignant proliferation of smooth muscle cells consistent with FNCLCC grade 2 leiomyosarcoma. The tumor was strongly positive for SMA and desmin, positive for vimentin and ß catenin and was negative for CK, CD 117, S 100, HMB 45 and LCA. Tumor cells showed 30% expression of Ki67. Immunihistochemical analysis was further confirmatory of leiomyosarcoma. Following histopathological examination and retrospectively evaluating the features on imaging, the possibility of primary leiomyosarcoma of infrarenal inferior vena cava was considered.
Patient then underwent laparotomy through midline approach for excision of the tumor. Intraoperatively, an exophytic circumscribed lobulated soft tissue tumor was seen arising from the anterior wall of infrarenal inferior vena cava. No intraluminal component was noted. Bilateral renal vessels were unremarkable. The tumor along with the corresponding segment of the inferior vena cava were excised and inferior vena cava graft reconstruction was performed. Bilateral renal veins were anastamosed to the reconstructed inferior vena cava. Excisional biopsy further confirmed the diagnosis.
Discussion
Leiomyosarcoma is the most common primary tumor of the inferior vena cava. Incidence is higher in females between 5th and 6th decade of life [5]. It is a slow growing plastic tumor that extends along the tissue planes of least resistance than directly invading the adjacent structures [7]. It generally presents as a retroperitoneal lesion and is often confused with primary retroperitoneal tumors. Surgical resection is the primary therapeutic option. Imaging plays a crucial role in preoperative evaluation of retroperitoneal tumors, in lesion characterization and assessment of spread. Differentiating LMS of inferior vena cava from other primary retroperitoneal tumors is a diagnostic challenge but carries high clinical implications in surgical planning. Preoperative differentiation of LMS of inferior vena cava (IVC) origin from a primary retroperitoneal tumor compressing the IVC is critical because primary origin from IVC will require optimal surgical planning for vascular repair or reconstruction. LMS of inferior vena cava can be classified on the basis of the involvement of IVC and on the basis of anatomical location of the tumor.
Classification based on IVC involvement [7]:
• Extraluminal – an exophytic mass lesion arising along the vascular mural wall of IVC.
• Intraluminal – soft tissue tumor within the lumen of IVC, extending along its course with high probability of extending into the right atrium.
• Mixed – shows both intraluminal and extraluminal components.
Classification based on anatomical location [8]:
• Segment I (Infra renal): tumor along the infrarenal part of IVC, commonly presents with pedal edema or back ache.
• Segment II (Inter renal or supra renal): tumor is seen at the origin of renal veins or above it without obvious involvement of the hepatic veins, presents with pain abdomen and renovascular hypertension.
• Segment III (Suprahepatic): involvement of hepatic veins with or without extension into the right atrium, presents as abdominal distension, hepatomegaly, vomiting, ascites and jaundice. Rarely presents with Budd–Chiari syndrome.
Common radiological features that aid in characterization of primary LMS of inferior vena cava in light of the existing literature are enumerated in table 1. In a large pooled analysis of 377 cases by Wachtel H et al (12), 133 (59.1%) cases were extraluminal, 56 (24.9%) were intraluminal and 36 (16%) cases had both intra- and extraluminal components. Similar results were reported by Mingoli et al (5), where out of a total of 144 cases, 106 (73.6%) cases had predominantly extraluminal tumor and 31 (21.5%) had intraluminal involvement. Tumors with intra- and extraluminal components are referred to as dumbbell tumors. Predominant intraluminal tumors and mixed tumors usually extend distally or proximally along the vascular lumen while extraluminal tumors generally show restricted or no extension as reported in our case. These tumors occur at a higher frequency in segment 2 of the IVC (i.e. between the origin of renal veins and confluence of hepatic veins) followed by segment 1 (infrarenal IVC) [5, 12, 14]. Tumors epicentered in the segment 2 had relatively higher incidence of extension into the hepatic veins and renal veins [3, 14, 17, 20]. Tumors originating from the suprahepatic segment (segment 3) of IVC commonly have a predominantly intraluminal component and show high prevalence of intracardiac extension [5, 13, 18]. Gafarli S et al reported an intraluminal tumor in segment 1 with transluminal extension into the right atrium, presenting with pulmonary thromboembolism [21]. Generally, intraluminal tumors from segment 1 show distal extension in to the iliac veins [5, 15]. Cortecero JM et al [13] reported an intraluminal leiomyosarcoma involving all three segments of the IVC extending into the right atrium and into the pelvis along right ovarian vein. Extraluminal LMS usually shows preserved interfaces with the adjoining organs. However, infiltration into the adjacent structures is not uncommon. Owing to the proximity of IVC to aorta, encasement of aorta is seen in aggressive tumors [5, 17, 19]. Extraluminal and mixed tumors with extraluminal component can also involve renal parenchyma, renal vascular pedicle and hepatic parenchyma (if occurring on right side) [9, 15, 17, 20]. Involvement of adrenal gland in a mixed tumor has also been reported earlier [14]. In 2004, Shvarts O et al [9] presented a mixed LMS of suprarenal (segment 2) IVC with extensive involvement of the right kidney that was initially misdiagnosed as a primary renal tumor. Li A et al [26] reported a case of large extraluminal LMS of segment 2 of the IVC that mimicked primary hepatocellular carcinoma with intravascular tumor thrombus. However in our case, fat planes with the adjacent structures were sharp with no obvious infiltration into surrounding structures.
Table 1. Salient radiological features for characterization and evaluation of leiomyosarcoma of the inferior vena cava from existing literature.
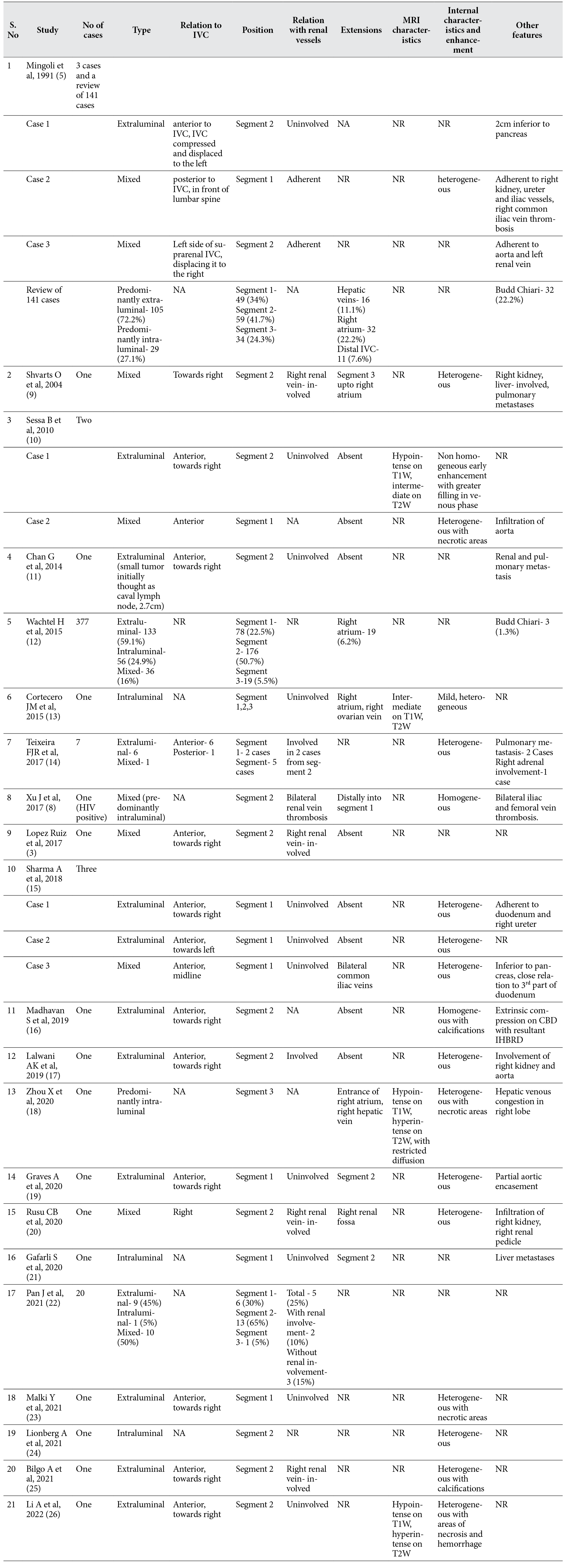
One of the important imaging feature for characterization of retroperitoneal tumors is the relationship with IVC. Webb Em et al [27] undertook a retrospective analysis of 13 cases of retroperitoneal tumors to study the performance of four different radiological signs in differentiating LMS of IVC from other primary retroperitoneal tumors. Radiological signs that were studied are positive embedded sign, negative embedded sign, imperceptible IVC sign and tumor in lumen. They observed that imperceptible IVC lumen at the site of maximum contact is highly predictive of a IVC origin. Case described in this manuscript had the imaging feature. Prone imaging can be of diagnostic utility in demonstrating primary origin from IVC in patients with predominantly extraluminal lesion. On prone imaging, these tumors show persistent contact with the wall of IVC or persistent nonvisualization of the lumen of IVC. Tumors with predominant extraluminal component commonly occur in anterior relation to the IVC and towards the right side of midline [9-11, 17, 19]. However, tumors originating posteriorly from the IVC have also been reported [5, 14].
These tumors commonly show heterogeneous enhancement with areas of necrosis within [9, 14, 15]. Sessa B et al [10] reported inhomogeneous early enhancement followed by homogenous filling in portal venous phase. We observed inhomogeneous contrast uptake in arterial phase followed by near homogeneous enhancement in portal venous and delayed phases with nonenhancing areas within. Few authors have also reported presence of calcification [16, 25] and hemorrhage [26] within the lesion.
On MR imaging LMS of IVC appears hypo- to intermediately intense on T1W and hyperintense on T2W [10, 18, 26]. Due to their malignant nature, these tumors also show diffusion restriction [18]. Similar signal characteristics were observed in our case also.
On Positron emission tomography (PET), these tumors show mild to intense uptake of fluorodeoxyglucose (FDG) [21, 28, 29]. Singh N et al [28] presented a case of intraluminal LMS showing intense FDG uptake with SUVmax value of 18. In the above presented case, the lesion showed mild FDG uptake with an SUVmax value of 4. PET CT has an indispensable role in the evaluation of intraluminal tumors in differentiating tumor thrombus from bland nontumor thrombus since these tumors present with venous thrombosis. Another significant role of PET CT lies in assessment of spread. These tumors can be locally aggressive but distant metastases are uncommon. However, lung is the most common site of metastasis [2, 11, 14]. Metastasis to the liver and kidney have also been reported [11, 21].
Important differential diagnoses include retroperitoneal sarcoma and lymphoma. Lymphomas show distinctive imaging features such as homogeneous enhancement, hypointense signal on T1W and T2W showing intense diffusion restriction and avid FDG uptake. Differentiating primary retroperitoneal sarcoma from leiomyosarcoma of the IVC is challenging. However, absence of perceptible lumen of IVC at the site of maximum contact and persistent contact with the wall of IVC on prone imaging are strong predictors of IVC leiomyosarcoma.
Histopathological sampling of the tumor is performed under ultrasonographic or computed tomographic guidance. In patients with intraluminal extension of the tumor, few authors have demonstrated conventional cavagraphy based transvenous sampling [8, 24].
En bloc surgical excision with negative surgical margins remains the main stay of treatment. Being tumors of vascular origin, these tumors require vascular repair in form of primary repair or graft based on the size and location of the tumor. Achieving clear surgical margin is the most significant factor affecting survival [30]. Patients with features of advanced disease undergo palliative chemotherapy and radiotherapy. Patients with clear resection margins are also treated with adjuvant chemotherapy and radiation therapy. However, the benefit of adjuvant chemoradiation is unclear [14].
Conclusions
Leiomyosarcoma of the inferior vena cava is a rare tumor of vascular origin. Imaging plays an imperative role in the diagnosis and preoperative evaluation. These tumors commonly show heterogeneous enhancement and occur in the segment 2 of IVC at a higher frequency. Extraluminal form is common. PET CT plays a prime role in the diagnosis of intraluminal form of the tumor.
Conflicts of interest
The Autrhors confirm that this work is original and has been published only in a pre-print service (www.researchsquare.com), the DOI is the following: https://doi.org/10.21203/rs.3.rs-1918169/v1.
References
- Perl L. Ein fall von sarkom der vena cava inferior. Virchows Arch. 1871;53:378– 83.
- Bower TC, Stanson A. Diagnosis and management of tumors of the inferior vena cava. In: Rutherford RB, ed. Vascular surgery. WB Saunders; 2000:2077–92.
- Lopez Ruiz JA, Tallon Aguilar L, Marcenco-de Cuadra B, Lopez- Perez J, Oliva- Mompean F, Padillo- Ruiz J. Leiomyosarcoma of inferior vena cava: Case report and literature review. Cir Cir. 2017;85(4):361–5. doi:10.1016/j.circir.2016.05.002.
- Shindo S, Matsumoto H, Ogata K, Katahira S, Kojima A, Iyori K et al. Surgical treatment of retroperitoneal leiomyosarcoma invading the inferior vena cava: report of three cases. Surg Today. 2002;32(10):929–33. doi: 10.1007/s005950200185.
- Mingoli A, Feldhaus RJ, Cavallaro A, Stipa S. Leiomyosarcoma of the inferior vena cava: Analysis and search of world literature on 141 patients and report of three new cases. J Vasc Surg. 1991;14(5):688–99. doi: 10.1067/mva.1991.30426.
- Mann GN, Mann LV, Levine EA, Shen P. Primary leiomyosarcoma of the inferior vena cava: A 2 institutional analysis of outcomes. Surgery. 2012;151(2):261–67. doi:10.1016/j.surg.2010.10.011.
- Cope JS, Hunt CJ. Leiomyosarcoma of inferior vena cava. AMA Arch Surg. 1954;68(6):752–56. doi: 10.1001/archsurg.1954.01260050754004.
- Xu J, Velayati A, Berger BJ, Liu M, Cheedella NKS, Gotlieb V. Leiomyosarcoma of the inferior vena cava in an hiv-positive adult patient: a case report and review of the literature. Am J Case Rep. 2017;18:1160–65. doi: 10.12659/ajcr.905787.
- Shvarts O, Han KR, Lam SJ, Belldegrun SA. Primary leiomyosarcoma of the inferior vena cava presenting as a renal mass. Rev Urol. 2004;39–42.
- Sessa B, Iannicelli E, Caterino S, D’Angelo F, Milione M, Ziparo V et al. Imaging of leiomyosarcoma of the inferior vena cava: Comparison of 2 cases and review of the literature. Cancer Imaging. 2010;10(1):80–84. doi: 10.1102/1470-7330.2010.0009.
- Chan G, Kroczak T, Drachenberg D. Leiomyosarcoma of the inferior venacava with renal metastasis: An unusual case and diagnostic challenge. Can Urol Assoc J. 2014;8(9-10):358–60. https://doi.org/10.5489/cuaj.2119.
- Wachtel H, Gupta M, Bartlett EK, Jackson BM, Kelz RR, Karakousis GC et al. Outomes after resection of leiomyosarcomas of the inferior vena cava: A pooled data analysis of 377 cases. Surg Oncol. 2015;24(1):21–27. doi: 10.1016/j.suronc.2014.10.007.
- Monteagudo Cortecero J, Guirau Rubio MD, Payá Romá A. Leiomyosarcoma of the inferior vena cava. Radiographics. 2015;35(2):616–20. doi: 10.1148/rg.352130105.
- Teixeira FJR, Netto SD, Perina AD, Torricelli FCM, Teixeira LR, Zerati AE, et al. Leiomyosarcoma of inferior vena cava: Survival rate following radical resection. Oncol Lett. 2017;14(4):3909–16. doi: 10.3892/ol.2017.6706.
- Sharma A, Ayappan MK, Raju R, Mathur K, Pawar P. Leiomyosarcoma of infrarenal inferior vena cava: A single institution experience and review of literature. Indian J Vasc Endovasc Surg. 2018;5(3):203–7.
- Madhavan S, Junnarkar PS, Koh NWC, Shelat GV. Inferior vena cava leiomyosarcoma in an octagenerian. Ann Hepatobiliary Pancreat Surg. 2019;23(3):274–77. doi: 10.14701/ahbps.2019.23.3.274.
- Lalwani AK, Ahuja J. Primary leiomyosarcoma of the inferior vena cava: Rare case report. J Dow Univ Health Sci. 2019;13(2):110–112. https://doi.org/10.36570/jduhs.2019.2.596.
- Zhou X, Wang M, Li S, Cai H, Liang L, Li ZP, et al. A case of a huge inferior vena cava leiomyosarcoma: Precise preoperative evaluation with Gadobutrol- enhanced MRI. Cancer Manag Res. 2020;12:7929–39. doi: 10.2147/CMAR.S258990.
- Graves A, Longoria J, Graves G, Ianiro C. Leiomyosarcoma of the inferior vena cava: A case report. J Surg Case Rep. 2020;(11):1–3. doi: 10.1093/jscr/rjaa479.
- Rusu CB, Gorbatai L, Szatmari L, Koren R, Bungargean CI, Feciche BO, et al. Leiomysarcoma of the inferior vena cava: Our experience and a review of the literature. Rom J Morphol Embryol. 2020;61(1):227–33. doi: 10.47162/RJME.61.1.25.
- Gafarli S, Igna D, Wagner M, Nistor A, Glanemann M, Stange B. Dyspnea due to an uncommon vascular tumor: Leiomyosarcoma of the infrahepatic vena cava inferior. Surg Case Rep. 2020;6(1):136. doi: 10.1186/s40792-020-00896-9.
- Pan J, Qiu C, He Y, Xue Y, Li D, Tian L, et al. A 10- year experience of leiomyosarcoma of the inferior venacava. PRE PRINT (version1) available at Research Square.
- Malki Y, Lazaar H, Bouhout T, Serji B, Benzirar A, Harroudi TE. Infrarenal venacava leiomyosarcoma treated with surgical resection and vascular reconstruction. Cureus. 2021;13(6):e15808. doi:10.7759/cureus.15808.
- Lionberg A, Ahmed O, Leef J. Leiomyosarcoma of the inferior vena cava. Appl Radiol. 2021. https://appliedradiology.com/communities/Vascular-IR-Imaging/leiomyosarcoma-of-the-inferior-vena-cava.
- Bilgo A, Saouli A, Zerda I, Zouaidia F, Karmouni T, El Khader K, et al. Leiomyosarcoma of the inferior vena cava with right renal invasion: About a case report. Afr J Urol. 2021;27:80. https://doi.org/10.1186/s12301-021-00189-z.
- Li A, Zhao T, Yin L, Chen K, Wu B, Wu M. Misdiagnosis of leiomyosarcomaof infrahepatic inferior vena cava as liver tumor in caudate lobe with inferior vena cava tumor thrombus. Ame J Surg CLin Case Rep. 2022;4:1–3.
- Webb EM, Wang ZJ, Westphalen AC, Nakakura EK, Coakley FV, Yeh BM. Can CT features differentiate between inferior vena cava leiomyosarcomas and primary retroperitoneal masses? Am J Roentgenol. 2013;200(1):205–209. doi:10.2214/AJR.11.7476.
- Singh N, Shivdasani D, Karangutkar S. Rare case of primary inferior vena cava leiomyosarcoma on F-18 fluorodeoxyglucose positron emission tomography- computed tomography scan: Differentiation from nontumor thrombus in a background of procoagulant state. Indian J Nucl Med. 2014;29(4):246–48. doi:10.4103/0972-3919.142629.
- Reddy VP, Vanveldhuizen PJ, Muelebach GF, Dusing WR, Birkbeck JP, Williamson KS, et al. Leiomyosarcoma of the inferior vena cava: A case report and review of the literature. Cases J. 2010;3:71.
- Ruiz CS, Kalbaugh CA, Browder SE, McGinigle KL, Kibbe MR, Farber MA, et al. Operative strategies for inferior vena cava repair in oncologic surgery. J Vasc Surg Venous Lymphat Disord. 2020;8(3):396–404. doi:10.1016/j.jvsv.2019.09.012.
Abbreviations:
IVC – Inferior vena cava
LMS – Leiomyosarcoma
CT – Computed tomography
MRI – Magnetic Resonance Imaging
PET – Positron Emission Tomography
FDG – Fluoro deoxy glucose
SUV – Standard Uptake Value
FNCLCC – French federation of Cancer Centres Sarcoma Group
TRUFI – True Fast Imaging with steady-state free precession
DWI – Diffusion Weighted Imaging
ADC – Apparent Diffusion Coefficient
VIBE – Volumetric Interpolated Breath-hold Examination