Acta medica Lituanica ISSN 1392-0138 eISSN 2029-4174
2022. Online ahead of print DOI: https://doi.org/10.15388/Amed.2022.29.2.17
Use of Tranexamic Acid to Reduce PostOperative Bleeding in Orthopaedic Oncology
Lorenzo Andreani
ORCID: https://orcid.org/0000-0002-8268-6987
Department of Orthopaedics and Trauma Surgery, University of Pisa, Pisa, Italy
Andrea Del Chiaro
ORCID: https://orcid.org/0000-0003-1198-2698
Department of Orthopaedics and Trauma Surgery, University of Pisa, Pisa, Italy
Edoardo Ipponi*
ORCID: https://orcid.org/0000-0003-2107-6357
Department of Orthopaedics and Trauma Surgery, University of Pisa, Pisa, Italy
Federico Di Sacco
Department of Orthopaedics and Trauma Surgery, University of Pisa, Pisa, Italy
Martina Caterino
Department of Orthopaedics and Trauma Surgery, University of Pisa, Pisa, Italy
Rodolfo Capanna
ORCID: https://orcid.org/0000-0001-7904-1267
Department of Orthopaedics and Trauma Surgery, University of Pisa, Pisa, Italy
Abstract. Background: Orthopaedic oncology often causes major blood losses that may put at risk patients’ hemodynamic balance and their overall clinical stability. To this date, transfusion therapy still represents the pivotal treatment to counterbalance the reduction in hemoglobin levels which occur after surgery. Although effective, transfusions are expensive and inevitably associated with a number of complications and therefore other solutions, such as procoagulative drugs, could play an important role to prevent massive blood losses.
Material and methods: We reviewed the clinical intercourse of 37 patients who underwent major bone resection due to malignant tumors of the lower limb. Cases were divided in two different groups: group G1 consisting of 12 patients treated intraoperatively with tranexamic acid and group G2 which was made of 25 controls.
Results: On average, patients treated with tranexamic acid (G1) required transfusion of 3.9 concentrated blood cells units during surgery and 0.9 units during the postoperative course. Other patients (G2), for their part, required on average 3.1 units intraoperatively and 2.1 units postoperatively. No significant difference was found in intraoperative transfusion rate (p=0.402). Instead, postoperative transfusions were significantly less frequent for patients treated with tranexamic acid (p=0.023). None of the 12 patients treated with tranexamic acid had evidence of Deep Vein Thrombosis.
Conclusion: Our outcomes indicate that the use of TXA was effective in reducing blood losses also for major surgical interventions in orthopedic oncology.
Keywords: Coagulation, Oncology, Orthopedics, Sarcoma, Tranexamic acid, Transfusion.
Traneksamo rūgšties naudojimas pooperaciniam kraujavimui sumažinti ortopedinėje onkologijoje
Santrauka. Įvadas: Ortopedinė onkologija dažnai sukelia didelio kraujo kiekio praradimą, o tai pavojinga ne tik pacientų hemodinaminei pusiausvyrai, bet ir kelia pavojų jų bendram klinikiniam stabilumui. Kol kas transfuzinė terapija vis dar yra pagrindinis gydymas siekiant suteikti atsvarą pacientų hemoglobino lygiui po operacijos sumažėti. Kraujo perpylimas yra efektyvus, tačiau brangus ir neišvengiamai susijęs su daugybe komplikacijų. Tad reikia ir kitokių sprendinių, pavyzdžiui, skirti prokoaguliacinius vaistus, kurie padėtų išvengti didžiulio kiekio kraujo netekimo.
Medžiagos ir metodai: Tyrėme 37 pacientų, kuriems buvo atlikta stambaus kaulo rezekcija dėl apatinių galūnių piktybinių navikų, gydymo klinikinę eigą. Pacientai buvo padalyti į dvi grupes: G1 grupė – 12 pacientų, kuriems chirurginės operacijos metu buvo skiriama traneksamo rūgštis, ir G2 kontrolinė grupė (25 pacientai).
Rezultatai: Vidutiniškai G1 grupės pacientams, kuriems buvo skiriama traneksamo rūgštis, kraujo perpylimui buvo sunaudojama 3,9 vieneto koncentruotų kraujo ląstelių operacijos metu, o dar 0,9 vieneto koncentruotų kraujo ląstelių prireikė pooperacinio gydymo metu. Kitiems pacientams (G2 grupės) vidutiniškai reikėjo 3,1 vieneto šių ląstelių operacijos metu ir 2,1 vieneto pooperaciniu laikotarpiu. Nebuvo nustatyta reikšmingo skirtumo dėl perpylimo masto operacijos metu (p = 0,402). Tačiau pooperaciniu laikotarpiu pacientams, kuriems buvo skiriama traneksamo rūgštis, reikėjo statistiškai reikšmingai kur kas mažiau perpilti kraujo (p = 0,023). Nė vienam iš 12 pacientų, gydytų traneksamo rūgštimi, nebuvo nustatyta, kad būtų pasireiškusi giliųjų venų trombozė.
Išvada: Tirtų atvejų baigtis rodo, kad yra efektyvu naudoti traneksamo rūgštį sumažinti prarandamo atliekant stambaus masto chirurgines intervencijas ortopedinėje onkologijoje kraujo kiekį.
Raktažodžiai: koaguliacija, onkologija, ortopedija, sarkoma, traneksamo rūgštis, kraujo perpylimas
_____________
* Corresponding author: Edoardo Ipponi, Department of Orthopaedics and Trauma Surgery, University of Pisa. Via Paradisa 2, Pisa, Italy, 56124. Phone: +39 3386381712, Fax: 050993415. Email: lorenzo.andreani.unipi@gmail.com
Received: 13/10/2022. Revised: 28/10/2022. Accepted: 21/11/2022
Copyright © 2022 Lorenzo Andreani, Andrea Del Chiaro, Edoardo Ipponi, Federico Di Sacco, Martina Caterino, Rodolfo Capanna. Published by Vilnius University Press.This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Tranexamic acid (TXA) is a synthetic molecule structurally analogous to the amino acid lysine. It fulfils its function by reversibly occupying the lysine binding site of the fibrinolytic enzyme Plasmin (Fig. 1). This bond continuously inactivates the enzyme and makes it unable to perform its normal activity, which is the clot lysis. The result is an antifibrinolytic procoagulative effect [1, 2].
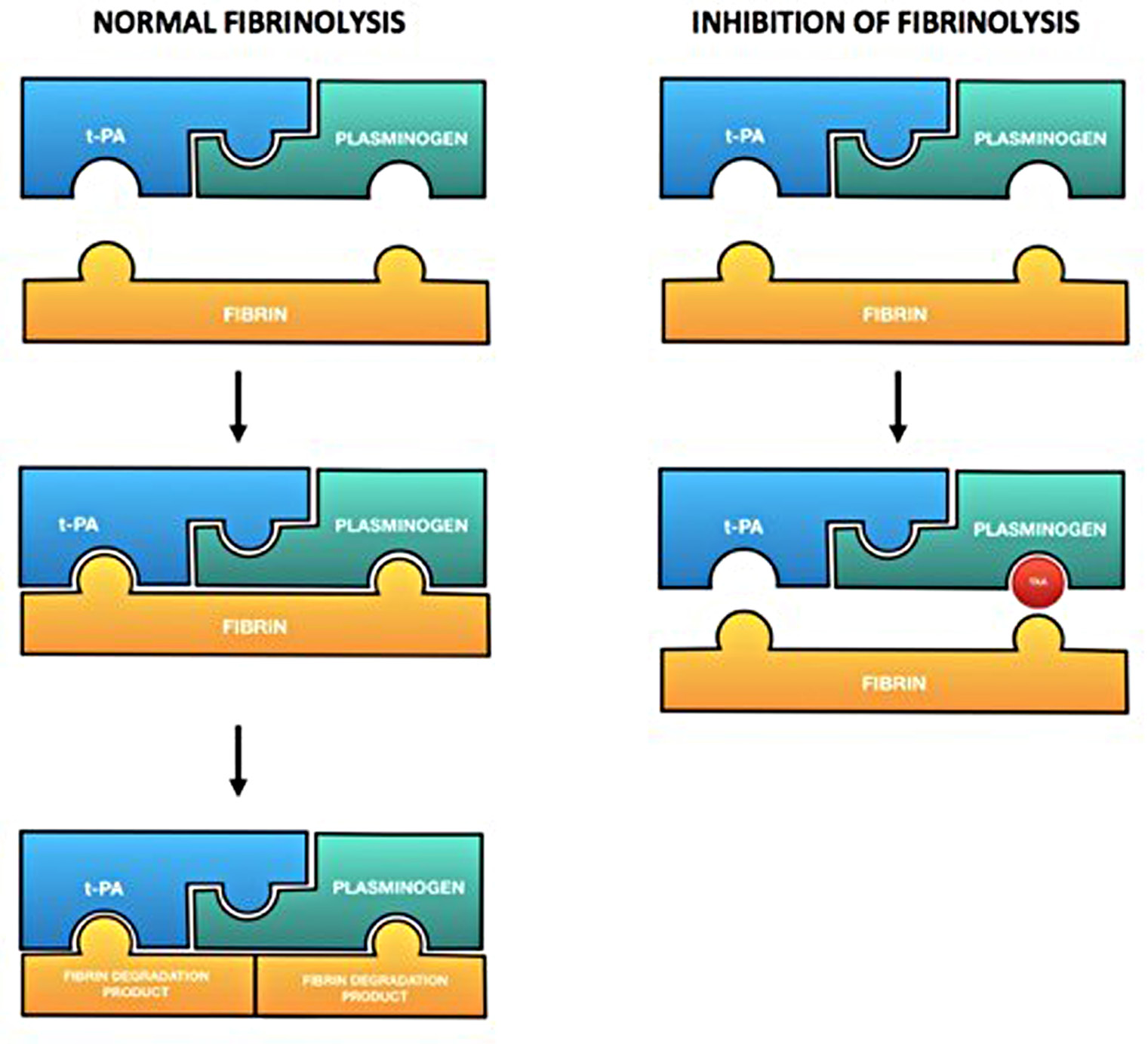
Figure 1. In normal fibrinolysis, plasminogen (linked with t-PA) binds and therefore degrades fibrin. Tranexamic acid (the red circle in the picture) occupies the binding site for fibrin and consequentially prevents its lysis.
Released from Shosuke Okamoto’s lab in the early 1960s, TXA was first prescribed to female patients with heavy menstrual blood losses and patients with hereditary coagulation disorders [2]. It did not take long before its indications were widened to elective surgery in consideration of its blood saving effects. Reduction of intraoperative and postoperative bleedings attributable to tranexamic acid led to a progressively wider use of the drug, not only in the obstetrical and gynecological field, but also in otolaryngology, cardiac surgery and general surgery [3-5].
In parallel, tranexamic acid progressively established itself as an alternative approach to minimize transfusion requirements also in major orthopedic surgery, alongside the other already existing methods such as blood salvage, controlled hypotension, hemodilution and stimulation of erythropoiesis with epoetin alfa. Total joint arthroplasty and spine surgery, for their nature associated with massive blood losses, first gave fertile field for the experimental use of TXA in orthopedics, drawing encouraging outcomes [6-9].
To this date, many studies have demonstrated the remarkable safety and efficacy of the drug, although the scientific community is yet to announce unanimous guidelines to regulate its use for orthopedics or anesthesia [10-14].
Although the drug is contraindicated in patients with history of venous or arterial thrombosis, intrinsic risk for thrombosis or thromboembolism, acute renal failure, subarachnoid hemorrhage and history of seizures and is suspected to actively increase the risk of thrombosis, several studies demonstrated good results in terms of reducing postoperative bleeding and therefore limiting the need of transfusion therapy [11-15].
Transfusion treatment is often necessary to maintain a good blood balance in patients who underwent major surgery and nowadays represent a relatively safe procedure thanks to all the controls in terms of microscopical composition, antigenic characteristics and microorganism detection. Nevertheless, their administration is inevitably associated with low but actual risks of developing fever, allergic reactions or infective diseases [6, 16, 17]. A larger use of tranexamic acid could theoretically reduce the transfusion, furtherly decreasing the number of those adverse events and reducing hospitalization costs. TXA could also reduce the days of hospitalization itself, allowing earlier discharge in light of a prompt and long-lasting stabilization of blood count.
For these reasons, tranexamic acid has been proposed and used in different branches of orthopedic surgery. Among them, orthopedic oncology represents one of the areas that could benefit the most from this therapy [10-14]. Systemic use of tranexamic acid has the potential to open a new horizon in blood loss control for patients treated with massive tumor resection by stabilizing their blood cells count and reducing their transfusion rate.
To this date, there is still paucity of studies about antifibrinolytic therapy to prevent massive intraoperative and postoperative hemorrhages in orthopedic oncology. In this paper we report our experience with tranexamic acid treatment during major interventions in orthopedic oncology.
Materials and methods
This single-center retrospective study was approved by our local ethics committee and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Our study consisted of a review of 37 patients treated with major bone resection due to malignant tumors of the lower limb in our institution between January 2016 and January 2020. Cases were divided in two different groups: group G1 consists of 12 patients treated with intraoperative use of tranexamic acid, while group G2 is made of 25 controls. During surgical procedure, patients belonging to G1 received an intravenous injection of tranexamic acid with a dose of 15 mg/kg. The administration was carried out 15 minutes before the beginning of the intervention in those surgeries which did not require pneumatic tourniquet; whereas in those who required the pneumatic tourniquet injection was made 15 minutes before exsufflation. Furthermore, 1000 mg of the drug, diluted in chloride solution and poured on gauzes, were used topically packing the wound for five minutes.
Each patient underwent deep venous thrombosis prophylaxis with enoxaparin 4000 IU once per day since the day before surgery.
Exclusion criteria for the use of tranexamic acid and the inclusion in G1 were known individual hypersensitivity to the drug, coagulation disorders, severe kidney damage (GFR < 30 ml/min/1.73m2), previous or present coronary artery disease, myocardial infarction, atrial fibrillation, stroke, arterial or venous thromboembolism.
For each patient we collected data regarding their age, gender, other personal data, anamnestic data, tumor type and site, reconstructive approach, but also the incidence of intraoperative and postoperative complications. Daily CBC and coagulation analysis were used during the entire hospitalization in order to assess the early systemic effects of tranexamic acid.
The effectiveness of our treatment with tranexamic acid was assessed counting the number of packed red blood cells units transfused during and after surgery in order to maintain patients’ hemodynamic situation acceptable and stable.
Each complication with grade II or higher according to the Clavien–Dindo classification potentially attributable to the use of tranexamic acid was reported.
Statistics. Statistical analysis was performed using Stata SE 13 (StataCorp LLC, College Station, TX). Statistical significance was set at 0.05 for all endpoints.
Results
Male to female ratio was 1.40 (7:5) in G1 and 1.28 (14:11) in G2, with an overall value of 1.31 (21:16). Mean age at surgery was 50.2 (24–78) years, 47.2 (24–71) for G1 and 53.1 (27–78) for G2.
Involved bones were pelvis in 9 cases, proximal femur in 18 cases, distal femur in 5 cases, the whole femur in one single case and proximal tibia in the remaining 4. Distribution in detail is reported in Table 1.
In our population 15 patients were suffering from secondary bone lesions, while the remaining 22 had primary bone tumors. Among these latter, 12 were diagnosed with chondrosarcoma, 7 with osteosarcoma, 2 with malignant fibrous histiocytoma and 1 Ewing sarcoma. Schematized histological nature is shown in Table 2.
Table 1. Age at surgery, gender ratio, tumor site and major coagulative complications for all patients in the study.
|
TOTAL |
G1 (Treated with TXA) |
G2 (Not treated with TXA) |
|
|
AGE AT SURGERY (y) |
50,2 (24-78) |
47,2 (24-71) |
53,1 (27-78) |
|
GENDER RATIO (m:f) |
1.31 (21:16) |
1.40 (7:5) |
1.28 (14:11) |
|
SITE |
|||
|
Pelvis |
9 |
3 |
6 |
|
Proximal Femur |
18 |
5 |
13 |
|
Distal Femur |
5 |
2 |
3 |
|
Whole Femur |
1 |
0 |
1 |
|
Proximal Tibia |
4 |
2 |
2 |
|
All |
37 |
12 |
25 |
|
COMPLICATIONS |
|||
|
Deep Vein Thrombosis |
1 |
0 |
1 |
(y) : years
(m:f) : males to females ratio
Table 2. Tumor type for each case in our population
|
TOTAL |
G1 (Treated with TXA) |
G2 (Not treated with TXA) |
|
|
Metastasis |
15 |
5 |
10 |
|
Osteosarcoma |
7 |
2 |
5 |
|
Chondrosarcoma |
12 |
4 |
8 |
|
Malignant Fibrous Histiocytoma |
2 |
1 |
1 |
|
Ewing Sarcoma |
1 |
0 |
1 |
|
TOTAL |
37 |
12 |
25 |
Reconstruction was carried out with mega-prostheses in 25 of our 37 patients. 7 patients required the implant of custom-made prostheses, whereas 5 were treated with bone allografts.
On average, patients treated with tranexamic acid (G1) required transfusion of 3.9 (2–5) units of concentrated blood cells during surgery and 0.9 (0–3) units during the whole postoperative course. Patients who were not treated with tranexamic acid (G2), for their part, required on average 3.1 (2–5) units intraoperatively and 2.1 (0–5) units postoperatively.
Whereas no significant difference was found in intraoperative transfusion rate (p=0.402), statistical analysis testified postoperative transfusions were significantly less frequent in those patients who were administered with intraoperative tranexamic acid (G1) (p=0.023).
Mean postoperative hospitalization lasted 7.9 (5–11) days for G1 cases and 8.7 (6–15) days for G2 cases. No significant correlation was evident between the use of tranexamic acid and hospitalization length.
None of the 12 patients treated with tranexamic acid had evidence of Deep Vein Thrombosis (DVT). Only 1 of our cases (2.7%), belonging to the G2 group and therefore not involved in the use of the drug, developed DVT after surgery. No case showed other major complications anyhow attributable to the coagulation process.
Discussion
Orthopaedic oncology is a surgical field that, for its own nature, is often associated with massive intraoperative and postoperative blood losses [18] (Fig. 2). On average, patients who suffer from a malignant tumor, especially in case of systemic spread of the disease, are exposed to a higher risk of hemorrhage compared to the general population [19-21]. This tendency is attributable to local anatomic and diffused metabolic variations induced by the neoplasm. Most of the malignant tumor masses in the bone are indeed characterized by intense angiogenesis and neovascularization to supply the intensely active tumoral cells with high volumes of circulating blood. The cut of these vessels in surgical theatre often leads to a profuse oozing that at times can be hard to cauterize. On a metabolic point of view sarcomas and other malignant tumors may also be associated with hypoproteinemia, lower levels of hemostatic and coagulation factors and platelet dysfunction: traits that together predispose to hypocoagulability [22, 23]. An additional incentive to this tendency consists in the ability of some tumoral cells to produce and expose on their surface enzymatic agents, such as tissue plasminogen activator or u-PA, capable of enhancing the degradation of fibrin [24, 25].
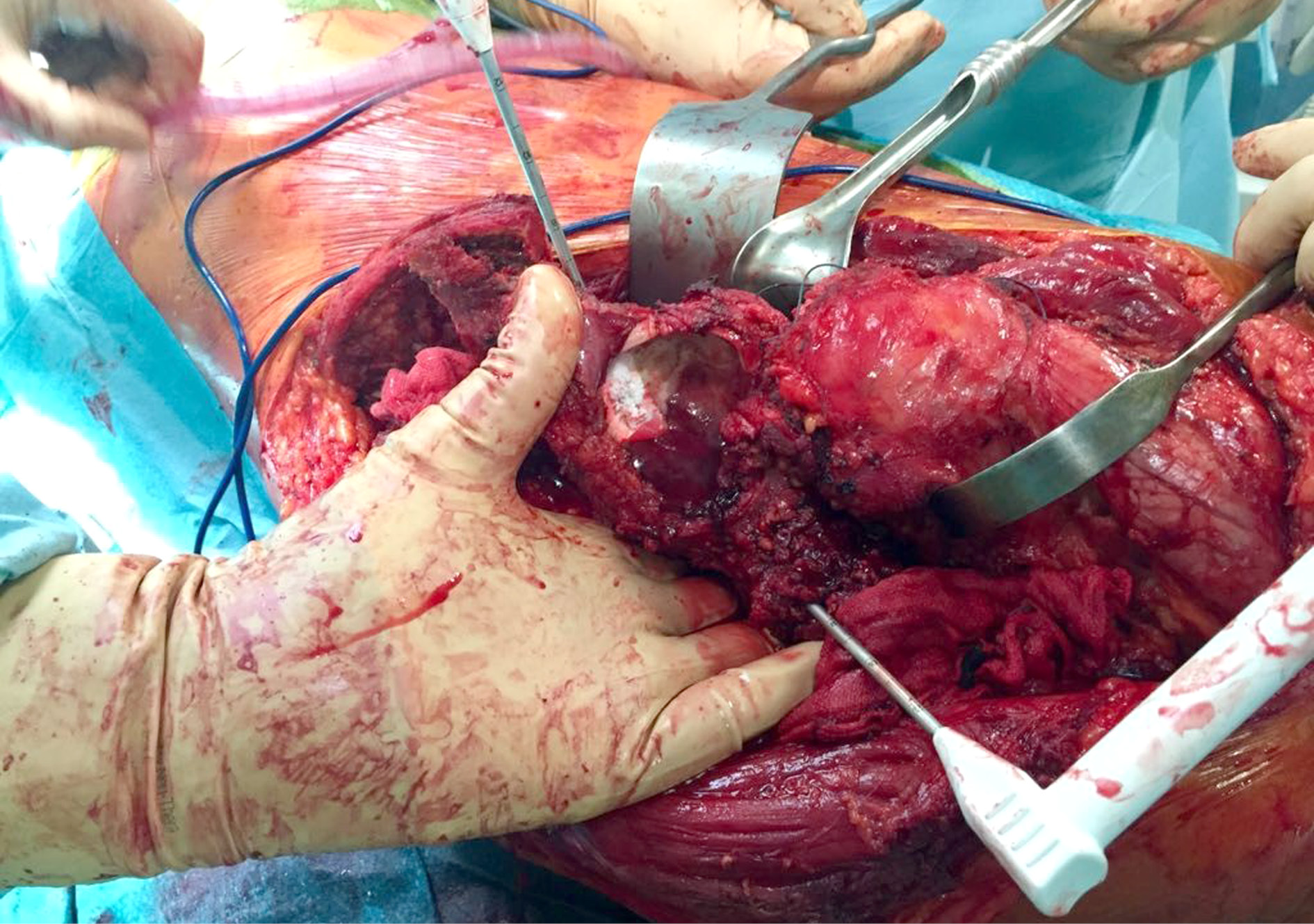
Figure 2. Surgical resection of a metastatic renal cell carcinoma involving the pelvis (exposed in the picture). An example of an orthopedic oncology intervention burdened by high risk of intra-operative blood losses.
Coagulation apart, a further concern with oncologic cases candidate for surgery is their frequent pre-existing low levels of haemoglobin. Anemia may be attributable to continuous blood dripping, bone marrow infiltration, nutritional deficiencies but also to the metabolic changes induced by the tumor. An important role is to be credited to the huge secretion of cytokines, including IL-6, which inhibit the production of erythropoietin and reduce the intracellular intake of iron molecules [26-28]. These criticalities could be made even more evident for those cases treated with chemotherapy due to its myelosuppressive effects.
In consideration of all the aforementioned factors, massive intraoperative and postoperative bleeding are not uncommon in nowadays orthopaedic practice and transfusion therapy often becomes necessary. Administration of allogeneic blood inevitably exposes patients to a number of risks. Transmission of infectious disease, immune system sensibilization, intravascular hemolysis and acute kidney disease are all documented risks associated with red cells transfusions, although their incidence has been reduced due to the strict controls made on the blood unit before the treatment as well as on the patient both during and after the injection [6, 16, 17].
Furthermore, production and conservation of every single blood unit costs hundreds of dollars to the treating institution, often representing one of the main costs during the whole hospitalization [29].
In order to reduce the number of blood units transfused, thereby reducing their risks and the total cost of the therapy, we recommend the use of tranexamic acid in orthopaedic oncology.
Its effectiveness and reliability has already been widely described when used in other surgical fields [3-11].
Focusing on orthopaedic and oncologic surgery, Ackerman et al [30] reported 104 oncological cases treated with perioperative intravenous injections of 15mg/kg of TXA during orthopaedic procedures. In their experience the incidence of major complications was relatively low (17%) and significant postoperative bleeding and hemodynamic instability were extremely uncommon (3%).
In our work TXA was used not only systemically, but also on a local basis. In fact, besides the 15 mg/kg administered intravenously, an additional 1000 mg were placed locally on the surgical field during intervention. In our opinion this bimodal administration of the tranexamic acid represents a more reliable way to keep high drug concentrations during surgery and in the hours immediately subsequent. At the same time this administration protocol limits its levels in distant anatomical sites, thereby reducing the risk of intraoperative and postoperative complications attributable to its procoagulative effects. Our study – obtained on oncologic patients who underwent massive bone resections and subsequent prosthetics orprosthetics or graft reconstructions – is consistent with the outcomes obtained by Ackerman et al. On the other hand, the control group had significantly higher blood losses. Although there was no significant difference during the intraoperative phase in terms of blood units administered, which indeed were slightly more on average for patients who were not treated with TXA, in the postoperative phase our results suggest the drug has a role in increasing local coagulation and therefore reduce the tendency to hemorrhage. In fact, during the hours and days that followed surgery, patients who were not treated with TXA required on average more than twice the number of blood units in order to maintain their clinical and hemodynamic stability. Furthermore, these encouraging results obtained with tranexamic acid were not associated with any major complication attributable to the drug.
Our outcomes thus indicate TXA has good effectiveness in terms of reducing bone losses and low risks also for major surgical interventions in orthopaedic oncology. This promising risk-benefit ratio should encourage a greater consideration and a wider intraoperative use, both local and systemic intraoperative, of tranexamic acid in orthopaedic oncology for those patients who have no previous absolute contraindications. The administration of the drug could in fact help early blood count stabilization, reduce the number of transfusions with all the risks and the costs connected to them.
We acknowledge our study had some limitations. The rarity of malignant bone tumors did not allow us to operate on wider populations, which partially limited the statistical significance of some of the data associations we wanted to investigate at the beginning of our research. Another limitation is represented by the retrospective nature of our study, which did not permit the complete standardization of the postoperative procedures.
Beyond these limits, our outcomes suggest TXA could represent a good addition to the intraoperative protocols during major surgeries in orthopedic oncology in order to minimize intraoperative and postoperative blood loss.
Conclusion
In conclusion, tranexamic acid represents a reliable therapeutic option for major surgery in orthopaedic oncology. Local and intravenous administration of the drug gave good results in reducing postoperative bleeding of the surgical site, thereby maintaining high the volume of circulating blood and limiting the reduction of its hemoglobin levels. This theoretically translates to more stable postoperative clinical conditions and lower transfusion rate during patients’ hospitalization.
Patient consent
All patients gave written consent to the use of their data and the publication of the results.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- Hunt BJ. The current place of tranexamic acid in the management of bleeding. Anaesthesia. 2015 Jan;70 Suppl 1:50-3, e18. doi: 10.1111/anae.12910.
- Tengborn L, Blombäck M, Berntorp E. Tranexamic acid--an old drug still going strong and making a revival. Thromb Res. 2015 Feb;135(2):231–42. doi: 10.1016/j.thromres.2014.11.012.
- Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999 Jun;57(6):1005–32. doi: 10.2165/00003495-199957060-00017.
- Brenner A, Ker K, Shakur-Still H, Roberts I. Tranexamic acid for post-partum haemorrhage: What, who and when. Best Pract Res Clin Obstet Gynaecol. 2019 Nov;61:66–74. doi: 10.1016/j.bpobgyn.2019.04.005.
- Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clin Otolaryngol. 2016 Dec;41(6):771–776. doi: 10.1111/coa.12645.
- Melvin JS, Stryker LS, Sierra RJ. Tranexamic Acid in Hip and Knee Arthroplasty. J Am Acad Orthop Surg. 2015 Dec;23(12):732–40. doi: 10.5435/JAAOS-D-14-00223.
- Lin ZX, Woolf SK. Safety, Efficacy, and Cost-effectiveness of Tranexamic Acid in Orthopedic Surgery. Orthopedics. 2016 Mar-Apr;39(2):119–30. doi: 10.3928/01477447-20160301-05.
- Ramirez RJ, Spinella PC, Bochicchio GV. Tranexamic Acid Update in Trauma. Crit Care Clin. 2017 Jan;33(1):85–99. doi: 10.1016/j.ccc.2016.08.004.
- Haase DR, Templeton KJ, Rosenthal HG, Sweeney KR. Tranexamic Acid in Patients With Cancer Undergoing Endoprosthetic Reconstruction: A Retrospective Review. J Am Acad Orthop Surg. 2020 Mar 15;28(6):248–255. doi: 10.5435/JAAOS-D-18-00798.
- Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M, Boettner F, Memtsoudis SG. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014 Aug 12;349:g4829. doi: 10.1136/bmj.g4829.
- Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011 Jan;93(1):39–46. doi: 10.1302/0301-620X.93B1.24984.
- Xu Q, Yang Y, Shi P, Zhou J, Dai W, Yao Z, Zhang C. Repeated doses of intravenous tranexamic acid are effective and safe at reducing perioperative blood loss in total knee arthroplasty. Biosci Trends. 2014 Jun;8(3):169–75. doi: 10.5582/bst.2014.01063.
- Franchini M, Mengoli C, Marietta M, Marano G, Vaglio S, Pupella S, Mannucci PM, Liumbruno GM. Safety of intravenous tranexamic acid in patients undergoing majororthopaedic surgery: a meta-analysis of randomised controlled trials. Blood Transfus. 2018 Jan;16(1):36–43. doi: 10.2450//2017.0219-17.
- Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009 Mar;123(5):687–96. doi: 10.1016/j.thromres.2008.09.015.
- Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: Causes and treatment. Ann Neurol. 2016 Jan;79(1):18–26. doi: 10.1002/ana.24558.
- Rawn J. The silent risks of blood transfusion. Curr Opin Anaesthesiol. 2008 Oct;21(5):664–8. doi: 10.1097/ACO.0b013e32830f1fd1.
- Lavoie J. Blood transfusion risks and alternative strategies in pediatric patients. Paediatr Anaesth. 2011 Jan;21(1):14–24. doi: 10.1111/j.1460-9592.2010.03470.x.
- Damade C, Tesson G, Gilard V, Vigny S, Foulongne E, Gauthé R, Ould-Slimane M. Blood loss and perioperative transfusions related to surgery for spinal tumors. Relevance of tranexamic acid. Neurochirurgie. 2019 Dec;65(6):377–381. doi: 10.1016/j.neuchi.2019.05.003.
- Johnstone C, Rich SE. Bleeding in cancer patients and its treatment: a review. Ann Palliat Med. 2018 Apr;7(2):265–273. doi: 10.21037/apm.2017.11.01.
- Chen Y, Tai BC, Nayak D, Kumar N, Chua KH, Lim JW, Goy RW, Wong HK. Blood loss in spinal tumour surgery and surgery for metastatic spinal disease: a meta-analysis. Bone Joint J. 2013 May;95-B(5):683–8. doi: 10.1302/0301-620X.95B5.31270.
- Dixon E, Datta I, Sutherland FR, Vauthey JN. Blood loss in surgical oncology: neglected quality indicator? J Surg Oncol. 2009 Jun 15;99(8):508–12. doi: 10.1002/jso.21187.
- Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013 Feb;11(2):223–33. doi: 10.1111/jth.12075.
- Al-Samkari H, Connors JM. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv. 2019 Nov 26;3(22):3770–3779. doi: 10.1182/bloodadvances.2019000369.
- Mengele K, Harbeck N, Reuning U, Magdolen V, Schmitt M. Tumorassoziierte Prognosefaktoren der Plasminogenaktivator-Familie. Bestimmung und klinische Wertigkeit von u-PA, t-PA, PAI-1 und PAI-2 [Tumor-associated prognostic factors of the plasminogen activator family: determination and clinical value of u-PA, t-PA, PAI-1, and PAI-2]. Hamostaseologie. 2005 Aug;25(3):301–10. doi: 10.1055/s-0037-1619664.
- Praus M, Collen D, Gerard RD. Both u-PA inhibition and vitronectin binding by plasminogen activator inhibitor 1 regulate HT1080 fibrosarcoma cell metastasis. Int J Cancer. 2002 Dec 20;102(6):584–91. doi: 10.1002/ijc.10767.
- Aulbert E, Jakob M, Kurschel E. Die Anämie bei malignen Tumorerkrankungen. V. Die Beziehung zwischen der tumorbedingten Hypotransferrinämie und dem Grad der Anämie [Anemia in malignant tumor diseases. V. The relation of tumor-induced hypotransferrinemia and the degree of anemia]. Onkologie. 1989 Apr;12(2):81–9. doi: 10.1159/000216606.
- Morant R, Bacchus L, Meyer J, Riesen WF. Tumoranämie und Entzündungsmarker [Tumor-induced anemia and markers of inflammation]. Schweiz Med Wochenschr. 1994 Dec 17;124(50):2267–71.
- Fraenkel PG. Anemia of Inflammation: A Review. Med Clin North Am. 2017 Mar;101(2):285–296. doi: 10.1016/j.mcna.2016.09.005. Epub 2016 Dec 24.
- Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010 Apr;50(4):753–65. doi: 10.1111/j.1537-2995.2009.02518.x.
- Ackerman RS, Hirschi M, Trona N, Joyce DM, Evans T, Patel SY. Incidence of Thromboembolic Events in Oncology Patients Receiving Intraoperative Tranexamic Acid During Orthopedic Surgery: A Retrospective Review at a Comprehensive Cancer Center. A A Pract. 2020 Jan 15;14(2):63–66. doi: 10.1213/XAA.0000000000001129.