Acta medica Lituanica ISSN 1392-0138 eISSN 2029-4174
2022. Online ahead of print DOI: https://doi.org/10.15388/Amed.2022.29.2.18
Risk Factors of Chronic Kidney Disease after Partial Nephrectomy
Jurijus Makevičius*
Institute of Clinical Medicine, Faculty of Medicine, Clinic of Gastroenterology, Nephrourology and Surgery, Vilnius University, Vilnius, Lithuania
Beata Kirstukaitė
Faculty of Medicine, Vilnius University, Vilnius, Lithuania
Renata Komiagienė
Institute of Biomedical Sciences, Faculty of Medicine, Department of Radiology, Nuclear Medicine and Physics of Medicine, Vilnius University, Vilnius, Lithuania
Arūnas Želvys
Institute of Clinical Medicine, Faculty of Medicine, Clinic of Gastroenterology, Nephrourology and Surgery, Vilnius University, Vilnius, Lithuania
ORCID https://orcid.org/0000-0002-9778-9372
Feliksas Jankevičius
Institute of Clinical Medicine, Faculty of Medicine, Clinic of Gastroenterology, Nephrourology and Surgery, Vilnius University, Vilnius, Lithuania
Marius Miglinas
Institute of Clinical Medicine, Faculty of Medicine, Clinic of Gastroenterology, Nephrourology and Surgery, Vilnius University, Vilnius, Lithuania
ORCID https://orcid.org/0000-0002-0017-468X
Abstract. Background: In comparison with radical nephrectomy, partial nephrectomy (PN) is considered a better option for small renal mass surgery, because of optimal kidney tissue removal and parenchyma preservation. But there are patients with worsening postoperative renal function (RF) and chronic kidney disease (CKD) after PN. Therefore, the study aimed to evaluate and detect risk factors for CKD after PN.
Materials and Methods: A prospective observational study was conducted, which consisted of 91 individuals who received PN with warm ischemia and an estimated preoperative glomerular filtration rate (eGFR) ≥ 60 ml/min/1.72m2 without pathologic albuminuria. Preoperative and intraoperative factors like intraoperative hypotension (IOH), blood loss, and resected part volume were analyzed.
Results: At 6-month follow-up, 14 (15.4%) patients experienced postoperative CKD. After 12 months of follow-up, 15 (16.5%) patients had CKD. Patients with CKD had a lower preoperative eGFR than non-CKD group (69.0 vs 91.0 ml/min/1.72m2, p < 0.001), longer ischemia (20.0 vs 14.0, p = 0.002) and IOH time (40.0 (40.0; 47.5) vs 0.0 (0.0; 26.2) min, p < 0.001). Also, higher volumes of resected tumor part of kidney and removed parenchyma with higher glomerulosclerosis amounts (73.3% vs 14.5%, p = 0.009) were found in CKD group. Estimated blood loss of > 500 ml during PN was discovered to be the major risk factor for CKD development (OR 11.13, 95% CI 1.88–65.92, p = 0.008). Furthermore, volume of resected kidney part (OR 1.05, 95% CI 1.05–1.10, p = 0.033) and IOH time (OR 1.11, 95% CI 1.03–1.19, p = 0.005) were identified as risk factors for postoperative CKD.
Conclusions: Patients after PN are at an increased risk of CKD development. Most commonly, postoperative CKD occurs in the first 6 months after PN and appears stable after 12 months of follow-up. Blood loss > 500 ml during PN, IOH and volume of resected kidney part can have an impact on postoperative RF and increase the risk of CKD.
Keywords: chronic kidney disease, partial nephrectomy, risk factors, intraoperative hypotension, blood loss.
Lėtinės inkstų ligos rizikos veiksniai po inkstų rezekcijos
Santrauka. Įvadas: Dėl optimalaus inksto audinių pašalinimo ir parenchimos išsaugojimo inksto rezekcija (IR) laikoma geresniu pasirinkimu operuojant mažus inksto darinius. Tačiau yra pacientų, kuriems po IR blogėja inkstų funkcija (IF) ir atsiranda pooperacinė lėtinė inkstų liga (LIL). Šio tyrimo tikslas – įvertinti ir nustatyti LIL rizikos veiksnius po IR.
Metodika: Buvo atliktas perspektyvusis tyrimas. IR su išemija atlikta 91 pacientui, kurio glomerulų filtracijos greitis prieš operaciją (GFG) ≥60 ml/min/1,72 m2 ir nebuvo patologinės albuminurijos. Taip pat analizuoti iki operacijos buvę ir jos eigoje tokie veiksniai kaip intraoperacinė hipotenzija (IOH), kraujo netektis ir pašalintos inksto dalies tūris.
Rezultatai: Pooperacinė LIL išsivystė 14 (15,4 %) pacientų po 6 stebėjimo mėnesių. Po 12 stebėjimo mėnesių LIL diagnozuota 15 (16,5 %) pacientų. Palyginti su pacientais, kurie neserga LIL, pacientų, sergančių pooperacine LIL, GFG prieš operaciją buvo didesnis 91,0 vs 69,0 ml/min/1,72 m2, p < 0,001, ilgesnis išemijos (14,0 vs 20,0, p = 0,002) ir IOH laikas (0,0 (0,0; 26,2) vs 40,0 (40,0; 47,5) min, p < 0,001). Taip pat LIL grupės pacientų buvo didesnis darinių ir pašalintos inksto parenchimos tūris bei didesnis glomerulosklerozės kiekis (73,3 % vs 14,5 %, p = 0,009). Nustatyta, kad apskaičiuota kraujo netektis >500 ml (AR 11,13, 95 %, PI 1,88–65,92, p = 0,008) IR metu ir didesnis inksto rezekcijos tūris (AR 1,05, 95 %, PI 1,05–1,10, p = 0,033) bei IOH laikas (AR 1,11, 95 %, PI 1,03–1,19, p = 0,005) buvo pagrindiniai LIL išsivystymo rizikos veiksniai.
Išvados: Pacientams po IR išlieka LIL išsivystymo rizika. Dažniausiai LIL pasireiškia per pirmuosius 6 mėnesius po IR ir atrodo stabili po 12 mėnesių stebėjimo. IOH, kraujo netektis daugiau nei 500 ml IR metu bei pašalintos inksto dalies tūris gali turėti įtakos pooperacinei IF ir padidinti LIL riziką.
Raktažodžiai: lėtinė inkstų liga, inksto rezekcija, rizikos veiksniai, intraoperacinė hipotenzija, kraujo netektis.
_________
* Corresponding author: Jurijus Makevičius, Institute of Clinical Medicine, Faculty of Medicine, Clinic of Gastroenterology, Nephrourology and Surgery, Vilnius University, Vilnius, Lithuania. Email: mak.jurijus@gmail.com
Received: 28/10/2022. Revised: 17/11/2022. Accepted: 28/11/2022
Copyright © 2022 Jurijus Makevičius, Beata Kirstukaitė, Renata Komiagienė, Arūnas Želvys, Feliksas Jankevičius, Marius Miglinas. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Chronic kidney disease (CKD) is highly prevalent health issue that affects 13.4% of global population [1]. Arterial hypertension and diabetes mellitus are being considered as the main risk factors in the etiology of this disease, but there are other reasons determining the development of it [2]. Patients after partial (PN) or radical nephrectomy (RN) surgeries have a significantly higher risk of worsening of postoperative renal function (RF), which increases the risk of mortality and has a negative impact on the patient’s quality of life [3]. As a consequence, the main aim of PN becomes not only to ensure the best oncological outcomes but to preserve kidney function during the surgery as well [4]. In comparison with radical nephrectomy, PN is considered a better option for treatment of small mass renal tumor, because of optimal kidney tissue removal [5]. Clinical studies reveal that there are other modifiable factors. Intraoperative blood loss is a significant risk factor for CKD development after PN, but it is closely related to intraoperative hypotension (IOH) time [6]. IOH during PN is usually caused by general anesthesia and is beneficial during surgery by helping to control or even reduce intraoperative blood loss. At the same time, longer time spent in hypotension raises the risk for CKD development after the surgery because of the caused renal parenchyma ischemia, which has a significant negative impact on postoperative RF [3, 4, 7, 8].
The study aimed to evaluate and detect preoperative and intraoperative risk factors for CKD after PN.
Materials and Methods
A prospective observational study was conducted in accordance with the Declaration of Helsinki between January 2018 and December 2019. The study was approved by the Regional Biomedical Research Ethics Committee (approval number 158200-16-882-389) and the State Data Protection Inspectorate. The first part of this study was focused on risk factors of acute kidney injury (AKI).
The study population consisted of individuals over 18 years old who underwent open or laparoscopic PN with warm ischemia due to kidney masses with an estimated preoperative glomerular filtration rate (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) ≥ 60 ml/min/1.73 m2. The study excluded patients with abnormal albuminuria, hyperuricemia, hyperkalemia, with a history of AKI, CKD, kidney surgery, unregulated hypertension and diabetes mellitus. PN was performed to patients with renal mass ≤ 7 cm. The same protocol of treatment option was for all patients.
We collected clinical and laboratory data, including age, gender, body mass index (BMI), serum creatinine (sCr). R.E.N.A.L. nephrometry score of CT imaging was applied for renal masses before PN. R.E.N.A.L. scoring is based on five basic CT features of the kidney’s tumor. Radius in cm in any axis: ≤4 – 1 point, >4 but <7 – 2 points, ≥7 – 3 points. Exophytic/endophytic tumor location: ≥50% exophytic – 1 point, <50% exophytic – 2 points, 100% endophytic – 3 points. Nearness to the renal collecting system or renal sinus measured in mm as the shortest distance from the deepest point of the tumor: ≥7 – 1 point, >4 but <7 – 2 points, ≤4 – 3 points. Location relative to the renal poles entirely below the inferior pole or above the superior pole – 1 point, mass crosses the polar line – 2 points, >50% of mass lies across the polar line or is entirely between the polar lines or crosses the axial midline – 3 points. Nephrometry score grading: score of 4–6 – low complexity, score of 7–9 – moderate complexity, score of 10–12 – high complexity. Also, we analyzed surgery data like duration, ischemia and IOH times, resected kidney parts, and tumor volumes.
Ischemia time is the time of the patient’s renal artery clamp. Ischemia time classification: <10 min, 10–20 min, >20 min.
Collected intraoperative blood classification: ≤500 ml and >500 ml.
RF was classified preoperatively and 12 months after surgery by eGFR: G1: 90 ml/min, G2: 60–89 ml/min, G3, 30–59 ml/min, G4, 15–29 ml/min, and G5, 15 ml/min. CKD is defined as having an eGFR of less than 60 ml/min/1.73 m2 for more than 3 months.
Patients were divided into 2 groups according to postoperative CKD status after 12 months follow-up. First group included patients with postoperative CKD and second – patients without CKD.
The following tools were used for statistical analysis: R 4.0.2 (software environment for statistical computing), RStudio 1.3.959 (integrated development environment for R), SPSS Statistics 23, G*Power 3.1.9.4. The Shapiro–Wilk and Kolmogorov–Smirnov (K–S) tests were used to check the data for normality. Nominal and ordinal variables were characterized by percentages and frequencies.
Univariate analyses were performed with postoperative CKD as the dependent variable and sociodemographic factors (age, gender, BMI, Charlson comorbidity index (CCI), metabolic syndrome (MetS)), laboratory data (preoperative eGFR and urine albumin-creatinine ratio (uACR)), surgery data (ischemia and IOH times, blood loss, resected part, tumor and removed parenchymal volumes) as the independent variables, but only the statistically significant (p < 0.05) were shown.
In order to assess the statistically significant influence of relevant independent variables (age, gender, BMI, CCI, MetS, preoperative eGFR, ischemia and IOH times, blood loss, resected part, tumor and removed parenchymal volumes) on postoperative CKD, we created models based on logistic regression equations with optimization to evaluate the independent effects of each covariate by controlling the effects of other variables. The receiver operating characteristic (ROC) curve was used to detect the optimal cut-off value.
Fisher’s chi-squared test was used to assess the statistically significant difference among the independent groups. When the p-value < 0.05 and the statistical test power was equal to 0.95 (1 ß = 0.95), the relationship between variables was considered statistically significant.
Results
91 patients agreed to participate in the study. The demographic and clinical characteristics of operated patients are summarized in Table 1.
Table 1. Cohort study clinical perioperative, intraoperative and postoperative data
|
Variable |
Value |
non-CKD group |
CKD group |
p-value |
|
Age, years |
Median (IQR) |
62.0 (56.0; 69.0) |
73.0 (70.0; 75.0) |
< 0.001 |
|
Gender |
Female |
29 (38.2%) |
9 (60.0%) |
0.154 |
|
Male |
47 (61.8%) |
6 (40.0%) |
||
|
BMI, kg/m2 |
Median (IQR) |
28.4 (25.5; 31.3) |
28.7 (26.0; 29.0) |
0.556 |
|
CCI, score |
Median (IQR) |
4.0 (3.0; 5.0) |
5.0 (5.0; 6.0) |
0.001 |
|
R.E.N.A.L. score, points |
Median (IQR) |
7.0 (6.0; 8.0) |
7.0 (6.0; 7.0) |
0.452 |
|
Metabolic syndrome |
Yes |
44 (57.9%) |
14 (93.3%) |
0.008 |
|
No |
32 (42.1%) |
1 (6.7%) |
||
|
Partial nephrectomy |
Laparoscopic |
43 (56.6%) |
7 (46.7%) |
0.574 |
|
Open |
33 (43.4%) |
8 (53.3%) |
||
|
Ischemia time, min |
Median (IQR) |
14.0 (10.0; 18.0) |
20.0 (17.0; 21.0) |
0.002 |
|
Ischemia time classification, min |
<10 |
16 (21.1%) |
1 (6.7%) |
0.076 |
|
10-20 |
49 (64.5%) |
8 (53.3%) |
||
|
>20 |
11 (14.5%) |
6 (40.0%) |
||
|
Blood loss, ml |
Median (IQR) |
300.0 (200.0; 402.5) |
510.0 (430.0; 550.0) |
<0.001 |
|
Blood loss classification, ml |
≤500 |
69 (90.8%) |
6 (40.0%) |
<0.001 |
|
>500 |
7 (9.2%) |
9 (60.0%) |
||
|
Intraoperative hypotension time, min |
Median (IQR) |
0.0 (0.0; 26.2) |
40.0 (40.0; 47.5) |
< 0.001 |
|
Resected part volume, cm3 |
Median (IQR) |
40.2 (25.1; 80.2) |
101.6 (73.2; 125.8) |
< 0.001 |
|
Tumor volume, cm3 |
Median (IQR) |
19.4 (7.4; 50.5) |
63.0 (38.2; 76.8) |
< 0.001 |
|
Removed parenchymal volume, cm3 |
Median (IQR) |
19.4 (11.2; 27.8) |
34.6 (27.6; 43.7) |
< 0.001 |
|
Parenchymal loss |
<10 cm3 |
27 (35.5%) |
- |
< 0.001 |
|
34 (44.7%) |
5 (33.3%) |
|||
|
>20 cm3 |
15 (19.7%) |
10 (66.7%) |
||
|
AKI status after 48 hours |
Non-AKI |
53 (69.7%) |
- |
< 0.001 |
|
I stage |
23 (30.3%) |
13 (86.7%) |
||
|
II stage |
- |
2 (13.3%) |
||
|
CKD after 6 months |
No |
79 (100%) |
1 (6.7%) |
< 0.001 |
|
Yes |
- |
14 (93.3%) |
Abbreviations: AKI – acute kidney injury; CKD – chronic kidney disease; IQR – interquartile range; BMI –body mass index; CCI – Charlson comorbidity index; R.E.N.A.L. – renal masses nephrometry scoring system; eBlood loss – estimated blood loss. p-values calculated for comparison of non-CKD and CKD cohorts.
Of these, 14 (15.4%) patients had postoperative CKD after 6-month follow-up. 15 (16.5%) patients developed postoperative CKD after 12 months: fourteen – stage III and one – stage IV. All patients with CKD had postoperative AKI.
There were more CKD group patients with higher CCI score, MetS, estimated blood loss during PN, longer ischemia and IOH times. Also, we found that volumes of resected tumor part of kidney and removed parenchyma were higher in the CKD group.
The CKD patients included in the study were older and have a lower preoperative and postoperative eGFR than non-CKD patients, Figure 1.
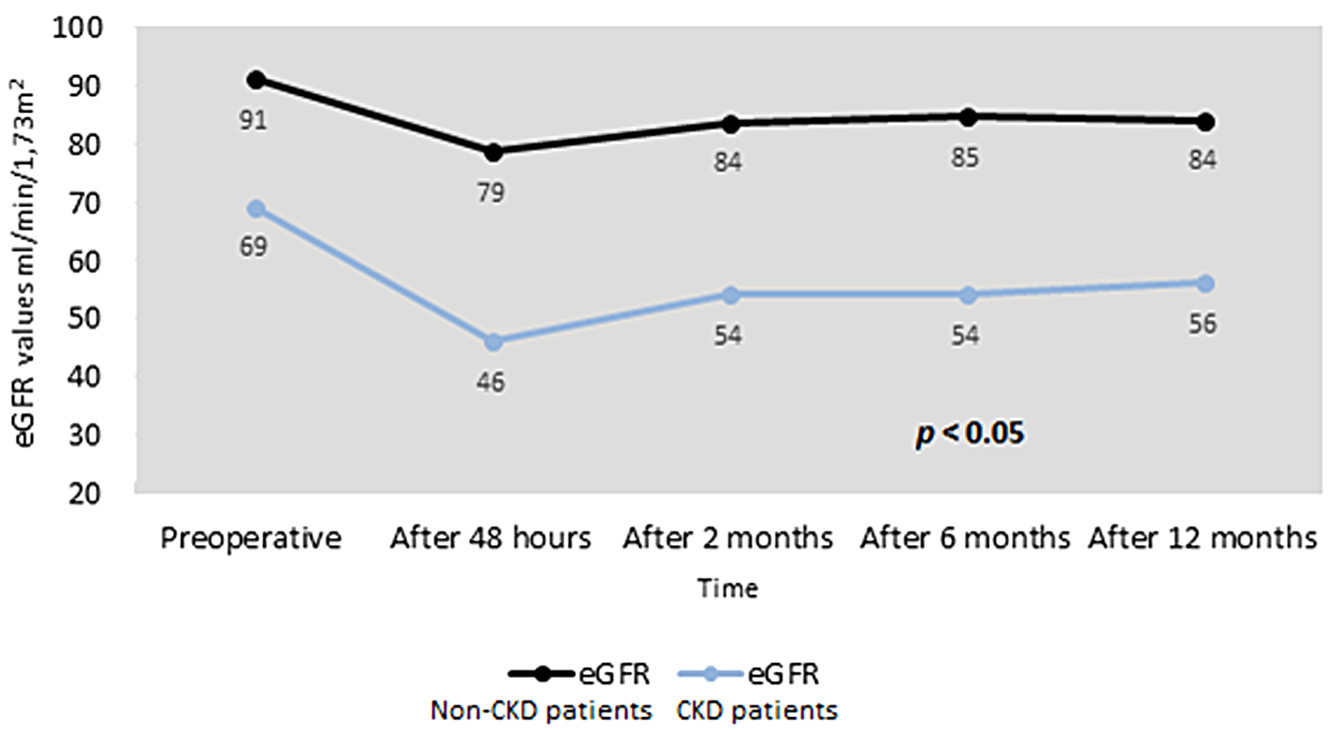
Figure 1. eGFR changes in CKD and non-CKD patients group.
Abbreviations: eGFR – estimated glomerular filtration rate. p-values calculated for comparison of non-CKD and CKD cohorts.
Clinical pathological analysis showed that CKD patients with removed kidney tissue had significantly more glomerulosclerosis changes, 73.3% vs 14.5%, p = 0.009.
The potential predictors of postoperative CKD after PN are summarized in Table 2.
Table 2. Associations between clinical characteristics and postoperative CKD persistence.
|
Univariate |
|||
|
Variables |
OR |
95% CI |
p - value |
|
Age |
1.19 |
1.09-1.34 |
0.001 |
|
CCI |
2.27 |
1.38-4.09 |
0.003 |
|
No metabolic syndrome |
0.10 |
0.01-0.53 |
0.029 |
|
Preoperative uACR |
3.24 |
1.59-7.80 |
0.003 |
|
Preoperative eGFR |
0.77 |
0.66-0.86 |
<0.001 |
|
Ischemia time |
1.17 |
1.06-1.33 |
0.006 |
|
Estimated blood loss > 500 ml |
14.79 |
4.21-57.65 |
<0.001 |
|
Hypotension time |
1.12 |
1.07-1.21 |
<0.001 |
|
Resected part volume |
1.01 |
1.00-1.03 |
0.016 |
|
Removed parenchymal volume |
1.05 |
1.02-1.08 |
0.003 |
Abbreviations: OR – odds ratio; CI – confidence interval; CCI – Charlson comorbidity index; uACR – urine albumin-creatinine ratio; eGFR – estimated glomerular filtration rate.
Age, CCI, higher preoperative uACR, estimated blood loss during PN, ischemia and intraoperative hypotension times, volumes of resected part and removed parenchyma were potential predictors of CKD development. However, patients without MetS and higher preoperative eGFR have a lower risk of postoperative CKD.
Estimated blood loss of > 500 ml during PN was found to be the major risk factor of CKD development (OR 11.13, 95% CI 1.88-65.92, p = 0.008) with volume of resected kidney part (OR 1.05, 95% CI 1.05-1.10, p = 0.005) and intraoperative hypotension time (OR 1.11, 95% CI 1.03-1.19, p = 0.005), Figure 2.
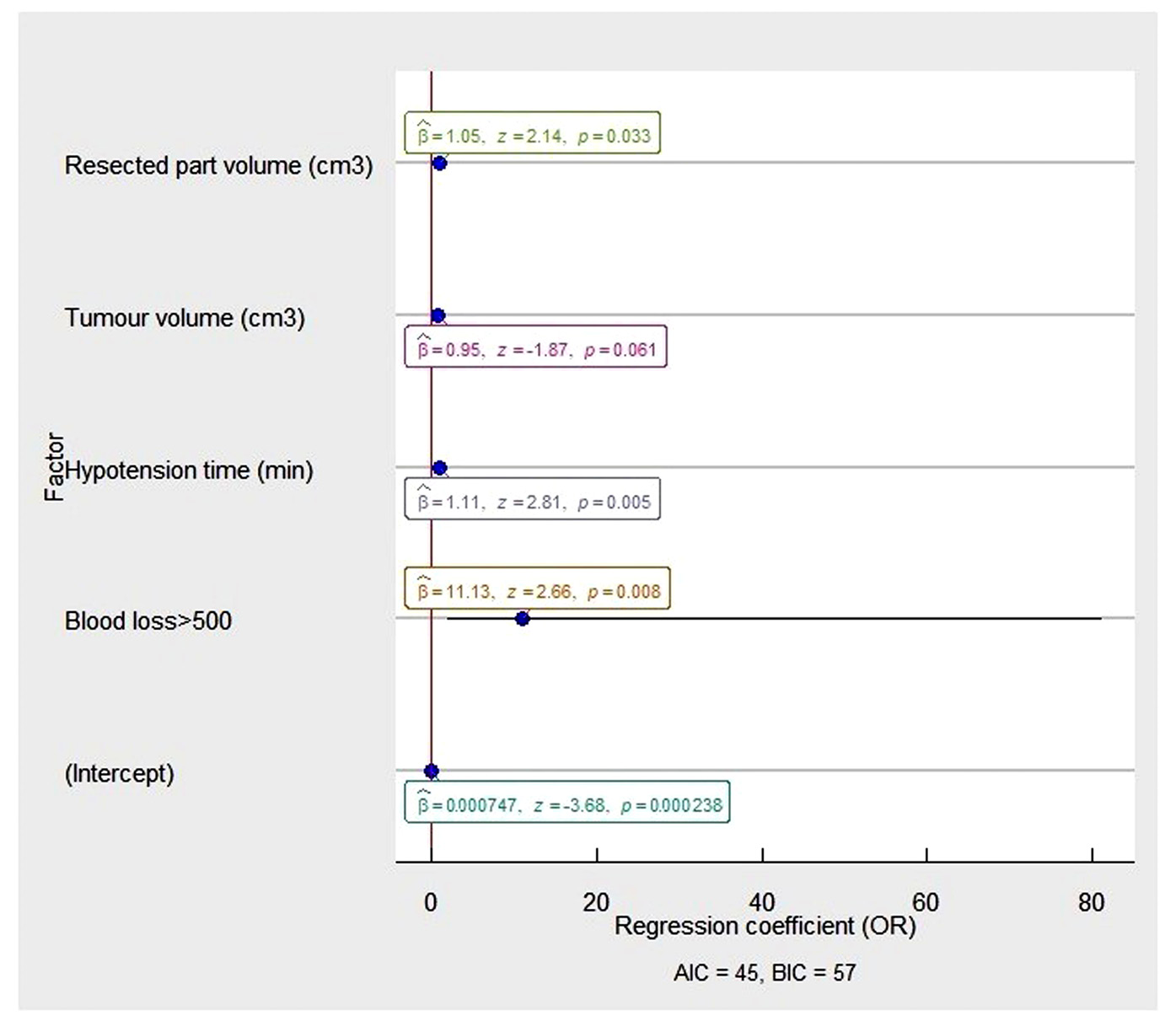
Figure 2. The multivariable analysis of risk factors associated with postoperative CKD in the patients after partial nephrectomy.
The OR and 95% CI were measured through logistic regression. Model characteristics: X = 46.87, P =.00; Pseudo-R² (Cragg–Uhler) = 0.68; Pseudo-R² (McFadden) = 0.58). Abbreviations: OR - odds ratio: AIC - Akaike information criteria; BIC – Bayesian information criteria.
In ROC analysis (Figure 3), estimated blood loss of more than 500 ml and volume of resected kidney part with IOH were the significant independent predictors (p < 0.0001) for postoperative CKD with an area under the ROC curve of 0.960 (95% CI = 0.921 to 0.999; sensitivity = 73.3%, specificity = 96.1%).
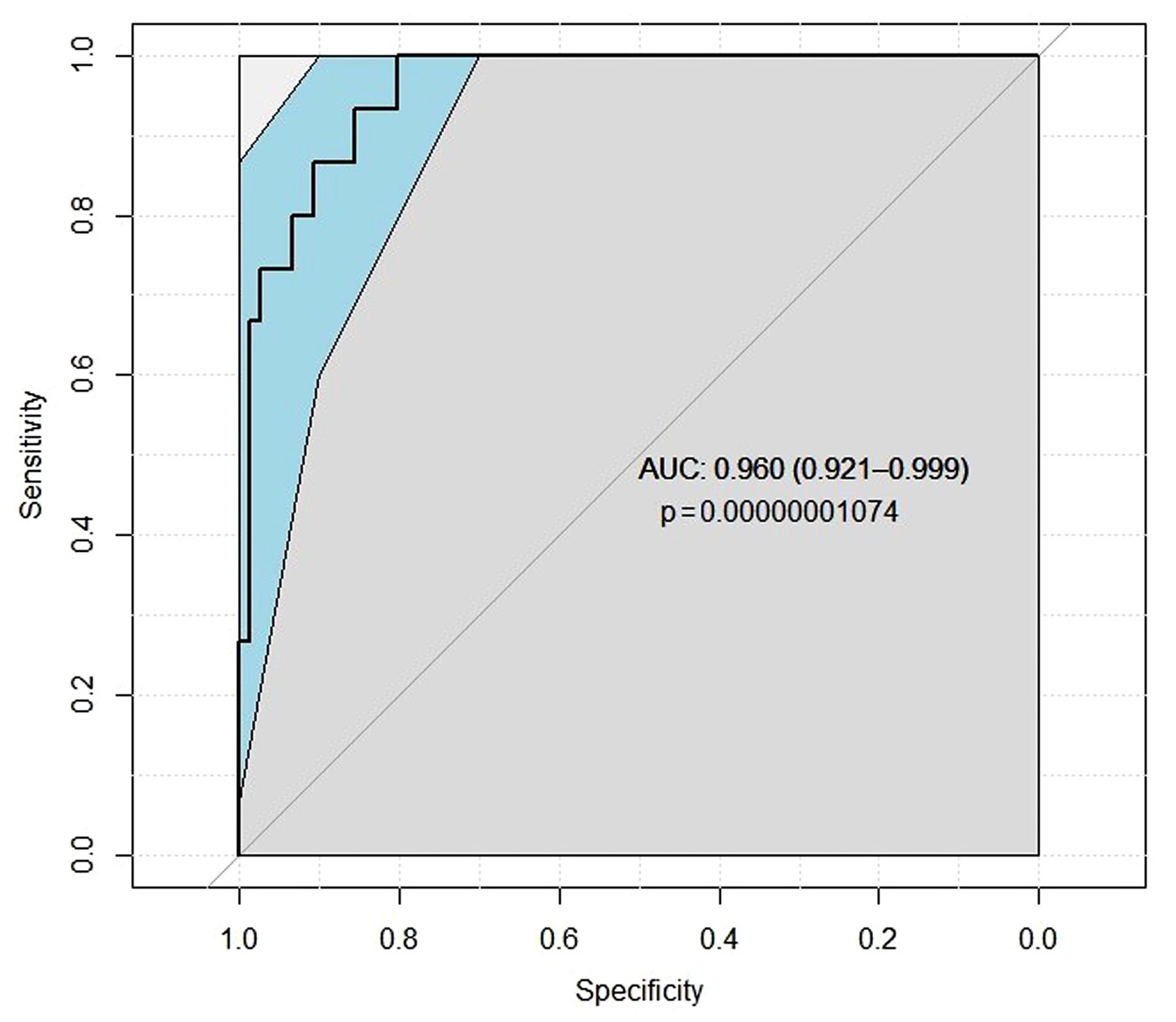
Figure 3. ROC analysis result detecting postoperative CKD.
Abbreviations: AUC – area under the curve.
All 91 patients with kidney tumor have a high cure rate with PN, without a recurrence rate and noncancer specific mortality after 1 year follow-up.
Discussion
Surgical treatment is considered to be the most effective treatment option for kidney cancer patients [9]. Comparing RN and PN, PN is considered as the most common approach for kidney cancer treatment as it is a nephron-sparing surgery. One of the main aims of kidney cancer surgeries is to preserve the maximum possible amount of unaffected renal parenchymal tissue [10]. Minimally invasive percutaneous treatment, such as cryoablation, radiofrequency ablation, microwave ablation are only alternative treatment options for older patients with detected small renal mass or patients that have contraindications for operation [9]. Indeed, choosing PN type of surgery is not enough to avoid CKD as a possible postoperative complication. Minimal distance between the surgical margin and tumor preserves surrounding healthy parenchyma and should improve RF, but there is a higher risk of attaining a positive margin and potentially worse oncologic outcomes. A study by Maurice et al. found a significant correlation between tumor size (p < 0.001) and higher lost parenchymal volume (p < 0.001). Excisional parenchymal loss increased by 7.1 cm2 for every 1 cm of tumor size (p < 0.01) [10].
Patients with small renal mass have a high prevalence of CKD even before PN because of common risk factors such as age, smoking, diabetes mellitus, and hypertension. After surgical intervention, the risk of new CKD diagnosis rises from 10% – 24% to 16% – 52.0% [11]. Our study examined age, CCI, MetS, uACR, estimated blood loss during PN, ischemia and IOH time, resected part and removed parenchymal volume as potential predictors of CKD, but only volume of resected kidney part, IOH time, and blood loss were found to be of clinical significance. Other clinical studies also show that RF preservation mostly relies on these modifiable surgical factors.
Studies that investigated volume of resected kidney part as a risk factor for worsening of RF came up with mixed results [10, 12–15]. A cohort study by Mir et al. confirmed that parenchymal volume loss plays an important role in avoiding renal decline after PN. The median preoperative eGFR in the operated kidney was 46 ml/min/1.73m2 and the median postoperative eGFR in the same kidney was 35 ml/min/1.73m2, which shows that the result of preserved eGFR was 79% [13].
A strong correlation between preserved eGFR and greater volume of saved parenchyma was found, whereas ischemia time had no correlation with RF preservation [13]. It is questionable if the amount of resected kidney parenchyma can be modifiable during PN because it is strongly influenced by tumor location and size. A study by Takagi et al. showed that the precision of tumor removal is modifiable and can be optimized in most cases. Overall, the median precision of performed surgeries was 93%, which proves that precise PN can be accomplished [14]. However, the only randomized clinical trial performed by the European Organization for Research and Treatment reported controversial results. The incidence of CKD was found to be similar in patients after RN and nephron sparing surgery, so they did not find any clinical significance of CKD risk due to surgical removal of nephrons [15]. Our study results show that volume of resected kidney part has a clinically significant impact on postoperative RF.
Chang et al. in a retrospective study compared robot-assisted and laparoscopic PN and reported a significant difference in blood loss during surgery. Robot-assisted PN was associated with intraoperative blood loss less in 24–50 ml compared to laparoscopic nephrectomy and in 39–94 ml compared to open PN. The same study showed that the incidence of CKD was significantly lower in robot-assisted nephrectomy compared to laparoscopic PN [16]. Our study showed that blood loss of over 500 ml is a significant risk factor for CKD. The same results published in the study of 1315 patients by Nientied et al. In the studied cohort, blood loss of 500 ml or more and perioperative blood transfusion were more frequently associated with reduced postoperative RF [17].
The worsening of RF after surgery is primarily due to the removal and devascularization of nephrons or incomplete renal parenchyma recovery from ischemia. Optimized PN includes precise excision of the tumor with high preservation of nephrons and complete kidney recovery from ischemia during the surgery [13]. Despite recent research indicating that hilar occlusion is not necessary, most PNs are performed with hilar occlusion to create a bloodless field [17, 18]. Ischemia damages kidney function because of vasoconstriction, tubular obstruction and reperfusion injury in the kidney [20]. Studies show that prolonged ischemia time quickly worsens postoperative RF. In a study of 362 kidneys, Thompson et al. also declared that warm ischemia time longer than 25 minutes is associated with the risk of new IV stage CKD cases increased by 2.3 times [21]. More recent studies show that RF decreases after an ischemia period of 20–25 minutes [17]. In the study by Mir et al., recovery from ischemia was defined as a percent of GFR divided by the percent of parenchymal mass. The median of recovery from ischemia was 95 % and 100 % for hypothermic cases and 92% for warm ischemia. Badly functioning kidneys recovered as well as highly functioning kidneys, and the recovery was proportionate to the nephron mass saved [13].
Hypotension is being considered as a common side effect of general anaesthesia that can cause kidney tissue ischemia [22]. Our definition of IOH is systolic blood pressure being lower than 90 mm Hg and diastolic blood pressure being lower than 60 mm Hg during general anaesthesia. Based on our multivariable analysis, hypotension during PN is found to be a statistically significant risk factor for worsening of RF with OR 1.11, p < 0.005. However, there is a lack of studies that investigate IOH during nephrectomy surgeries. A cohort study by Joosten et al. indicated that IOH is associated with AKI, which can be a cause of CKD development. 57% of the study patients had AKI stage 1–3. IOH was found to be short (defined as 8.6% of all intraoperative case time) in 52 (25%) patients, intermediate (defined as 8.6–39.5% of all case time) in 102 (50%) patients, and long (defined as more than 39.5% of all case time) in 51 (25%) patients. Results showed that patients who had short IOH times didn’t have AKI (84.6%), whereas the majority of the patients (86.3%) who had long IOH times had AKI. This study found that longer IOH time increases the risk of AKI [23]. Wesselink et al. declared IOH as the main cause of organ dysfunction after surgery and suggested that mean blood pressure (MAP) being lower than 80 mm Hg for longer than 10 minutes causes the occurrence of organ dysfunction [24]. Retrospective analysis by Sun et al. of 5127 patients undergoing noncardiac surgery showed the occurrence of AKI because of kidney ischemia in 6.3% of the studied patients and associated it with MAP being less than 60 mm Hg for 11 to 20 minutes and being less than 55 mm Hg for longer than 10 minutes [8].
This is the prospective study of independent risk factors for CKD, using modified intraoperative factors, such as hypotension and blood loss, during PN with warm ischemia. Our results allow to create a model to foreseen postoperative CKD. However, our study has some limitations. Firstly, the small size of patients, caused by follow-up in the local outpatient clinic far from the investigation centre, makes it difficult to determine which clinical and intraoperative factors impact postoperative CKD. Secondly, using a different IOH definition and a different examination method could have an impact on the total number of patients with IOH and final results. Furthermore, we examined patients after clamping the main artery during PN. In other cases, during PN, urologists frequently use cold ischemia, selective main tumor artery clamping, or enucleation techniques. Lastly, we follow-up and evaluate postoperative CKD after 12 months.
Conclusions
Patients after PN due to tumors are at an increased risk of CKD development. Postoperative CKD occurs most commonly in the first 6 months after PN and appears stable after 12 months of follow-up. Volume of kidney resected part, estimated blood loss of more than 500 ml, and IOH during PN are predictors of postoperative CKD.
Ethical Approval and Consent to Participate
The study was approved by the Regional Biomedical Research Ethics Committee (approval number 158200-16-882-389) and the State Data Protection Inspectorate. The study was conducted in accordance with the Declaration of Helsinki.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- Lv JC, Zhang LX. Prevalence and Disease Burden of Chronic Kidney Disease. In: Liu BC, Lan HY, Lv LL. eds. Renal Fibrosis: Mechanisms and Therapies. Vol 1165. Advances in Experimental Medicine and Biology. Springer Singapore; 2019:3–15. doi:10.1007/978-981-13-8871-2_1
- Charles C, Ferris AH. Chronic Kidney Disease. Prim Care Clin Off Pract. 2020;47(4):585–595. doi:10.1016/j.pop.2020.08.001
- Ellis RJ. Chronic kidney disease after nephrectomy: a clinically-significant entity? Transl Androl Urol. 2019;8(S2):S166–S174. doi:10.21037/tau.2018.10.13
- Rosiello G, Larcher A, Fallara G, et al. The impact of intraoperative bleeding on the risk of chronic kidney disease after nephron-sparing surgery. World J Urol. 2021;39(7):2553–2558. doi:10.1007/s00345-020-03504-5
- Dagenais J, Bertolo R, Garisto J, Chavali J, Kaouk J. “At‐risk” kidney: How surgical factors influence renal functional preservation after partial nephrectomy. Int J Urol. 2019;26(5):565–570. doi:10.1111/iju.13930
- Brignone J, Assersen KB, Jensen M, et al. Protection of kidney function and tissue integrity by pharmacologic use of natriuretic peptides and neprilysin inhibitors. Pflüg Arch - Eur J Physiol. 2021;473(4):595–610. doi:10.1007/s00424-021-02555-w
- Xiong L, Nguyen JK, Peng Y, et al. What Happens to the Preserved Renal Parenchyma After Clamped Partial Nephrectomy? Eur Urol. 2022;81(5):492–500. doi:10.1016/j.eururo.2021.12.036
- Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of Intraoperative Hypotension with Acute Kidney Injury after Elective Noncardiac Surgery. Anesthesiology. 2015;123(3):515–523. doi:10.1097/ALN.0000000000000765
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706–720. doi:10.1093/annonc/mdz056
- Maurice MJ, Ramirez D, Malkoç E, et al. Predictors of Excisional Volume Loss in Partial Nephrectomy: Is There Still Room for Improvement? Eur Urol. 2016;70(3):413–415. doi:10.1016/j.eururo.2016.05.007
- Hu SL, Chang A, Perazella MA, Okusa MD, Jaimes EA, Weiss RH. The Nephrologist’s Tumor: Basic Biology and Management of Renal Cell Carcinoma. J Am Soc Nephrol. 2016;27(8):2227–2237. doi:10.1681/ASN.2015121335
- Kotamarti S, Rothberg MB, Danzig MR, et al. Increasing Volume of Non-Neoplastic Parenchyma in Partial Nephrectomy Specimens Is Associated With Chronic Kidney Disease Upstaging. Clin Genitourin Cancer. 2015;13(3):239–243. doi:10.1016/j.clgc.2014.11.005
- Mir MC, Campbell RA, Sharma N, et al. Parenchymal Volume Preservation and Ischemia During Partial Nephrectomy: Functional and Volumetric Analysis. Urology. 2013;82(2):263–269. doi:10.1016/j.urology.2013.03.068
- Takagi T, Mir MC, Campbell RA, et al. Predictors of Precision of Excision and Reconstruction in Partial Nephrectomy. J Urol. 2014;192(1):30–35. doi:10.1016/j.juro.2013.12.035
- Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H. Renal Function After Nephron-sparing Surgery Versus Radical Nephrectomy: Results from EORTC Randomized Trial 30904. Eur Urol. 2014;65(2):372–377. doi:10.1016/j.eururo.2013.06.044
- Chang KD, Abdel Raheem A, Kim KH, et al. Functional and oncological outcomes of open, laparoscopic and robot-assisted partial nephrectomy: a multicentre comparative matched-pair analyses with a median of 5 years’ follow-up. BJU Int. 2018;122(4):618–626. doi:10.1111/bju.14250
- Nientiedt M, Bertolo R, Campi R, et al. Chronic Kidney Disease After Partial Nephrectomy in Patients With Preoperative Inconspicuous Renal Function – Curiosity or Relevant Issue? Clin Genitourin Cancer. 2020;18(6):e754–e761. doi:10.1016/j.clgc.2020.05.007
- Hung AJ, Cai J, Simmons MN, Gill IS. “Trifecta” in Partial Nephrectomy. J Urol. 2013;189(1):36–42. doi:10.1016/j.juro.2012.09.042
- Gill IS, Patil MB, de Castro Abreu AL, et al. Zero Ischemia Anatomical Partial Nephrectomy: A Novel Approach. J Urol. 2012;187(3):807–815. doi:10.1016/j.juro.2011.10.146
- Sciarra A, Von Heland M, Minisola F, Salciccia S, Cattarino S, Gentile V. Thulium Laser Supported Nephron Sparing Surgery for Renal Cell Carcinoma. J Urol. 2013;190(2):698–701. doi:10.1016/j.juro.2013.01.079
- Thompson RH, Lane BR, Lohse CM, et al. Renal Function After Partial Nephrectomy: Effect of Warm Ischemia Relative to Quantity and Quality of Preserved Kidney. Urology. 2012;79(2):356–360. doi:10.1016/j.urology.2011.10.031
- Südfeld S, Brechnitz S, Wagner JY, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57–64. doi:10.1093/bja/aex127
- Joosten A, Lucidi V, Ickx B, et al. Intraoperative hypotension during liver transplant surgery is associated with postoperative acute kidney injury: a historical cohort study. BMC Anesthesiol. 2021;21(1):12. doi:10.1186/s12871-020-01228-y
- Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706–721. doi:10.1016/j.bja.2018.04.036